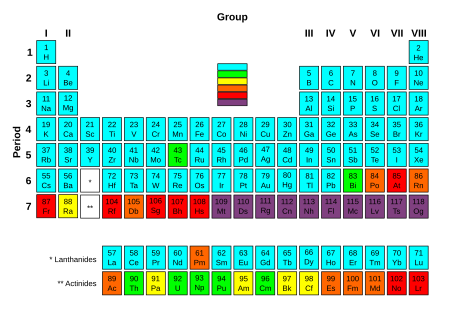
Actinide
humanoid or android), it has been argued for semantic reasons that actinium cannot logically be an actinoid, but IUPAC acknowledges its inclusion based on common usage.
[1][8] Nuclear weapons tests have released at least six actinides heavier than plutonium into the environment; analysis of debris from a 1952 hydrogen bomb explosion showed the presence of americium, curium, berkelium, californium, einsteinium and fermium.
The most abundant or easily synthesized actinides are uranium and thorium, followed by plutonium, americium, actinium, protactinium, neptunium, and curium.
The prevailing view that dominated early research into transuranics was that they were regular elements in the 7th period, with thorium, protactinium and uranium corresponding to 6th-period hafnium, tantalum and tungsten, respectively.
The first method is more important for applications, as only neutron irradiation using nuclear reactors allows the production of sizeable amounts of synthetic actinides; however, it is limited to relatively light elements.
[8] Actinium was discovered in 1899 by André-Louis Debierne, an assistant of Marie Curie, in the pitchblende waste left after removal of radium and polonium.
This view instead credits the 1902 work of Friedrich Oskar Giesel, who discovered a radioactive element named emanium that behaved similarly to lanthanum.
[33] It was first identified in 1913, when Kasimir Fajans and Oswald Helmuth Göhring encountered the short-lived isotope 234mPa (half-life 1.17 minutes) during their studies of the 238U decay chain.
They named the new element brevium (from Latin brevis meaning brief);[34][35] the name was changed to protoactinium (from Greek πρῶτος + ἀκτίς meaning "first beam element") in 1918 when two groups of scientists, led by the Austrian Lise Meitner and Otto Hahn of Germany and Frederick Soddy and John Arnold Cranston of Great Britain, independently discovered the much longer-lived 231Pa.
[39] Actinides with the highest mass numbers are synthesized by bombarding uranium, plutonium, curium and californium with ions of nitrogen, oxygen, carbon, neon or boron in a particle accelerator.
Thus nobelium was produced by bombarding uranium-238 with neon-22 as The first isotopes of transplutonium elements, americium-241 and curium-242, were synthesized in 1944 by Glenn T. Seaborg, Ralph A. James and Albert Ghiorso.
[44] Over the few years, milligram quantities of americium and microgram amounts of curium were accumulated that allowed production of isotopes of berkelium[45][46] and californium.
Instantaneous exposure of uranium-238 to a large neutron flux resulting from the explosion produced heavy isotopes of uranium, which underwent a series of beta decays to nuclides such as einsteinium-253 and fermium-255.
The discovery of the new elements and the new data on neutron capture were initially kept secret on the orders of the US military until 1955 due to Cold War tensions.
[9][52] Nevertheless, the Berkeley team were able to prepare einsteinium and fermium by civilian means, through the neutron bombardment of plutonium-239, and published this work in 1954 with the disclaimer that it was not the first studies that had been carried out on those elements.
247Bk is an alpha-emitter with a long half-life of 1,380 years, but it is hard to obtain in appreciable quantities; it is not formed upon neutron irradiation of plutonium because β-decay of curium isotopes with mass number below 248 is not known.
It is an α-emitter with a half-life of 20.47 days, a relatively weak γ-emission and small spontaneous fission rate as compared with the isotopes of californium.
The beta-minus decay, marked with an arrow pointing up-left, plays a major role for the balance of the particle densities of the nuclides.
The advantages of the sodium carbonate method are that the chemicals have low corrosivity (compared to nitrates) and that most non-uranium metals precipitate from the solution.
At high pH, this results in precipitation of diuranate, which is treated with hydrogen in the presence of nickel yielding an insoluble uranium tetracarbonate.
For example, the early-transition-metal-like prominence of the highest oxidation state, corresponding to removal of all valence electrons, extends up to uranium even though the 5f shells begin filling before that.
The unusually low melting point of neptunium and plutonium (~640 °C) is explained by hybridization of 5f and 6d orbitals and the formation of directional bonds in these metals.
[72] Typically, uranium nucleus is divided into two fragments with the release of 2–3 neutrons, for example: Other promising actinide isotopes for nuclear power are thorium-232 and its product from the thorium fuel cycle, uranium-233.
The rate of nuclear reaction is controlled by introducing additional rods made of boron or cadmium or a liquid absorbent, usually boric acid.
Mg-Th alloys are light and strong, but also have high melting point and ductility and thus are widely used in the aviation industry and in the production of missiles.
[112] The relative content of thorium and uranium isotopes is widely used to estimate the age of various objects, including stars (see radiometric dating).
[118] Plutonium-238 is potentially more efficient isotope for nuclear reactors, since it has smaller critical mass than uranium-235, but it continues to release much thermal energy (0.56 W/g)[111][119] by decay even when the fission chain reaction is stopped by control rods.
Its high specific energy (14.5 W/g) and the possibility of obtaining significant quantities of thermally stable compounds are attractive for use in long-lasting thermoelectric generators for remote use.
[30] Development of self-glowing actinide-doped materials with durable crystalline matrices is a new area of actinide utilization as the addition of alpha-emitting radionuclides to some glasses and crystals may confer luminescence.
[61][page needed] Plutonium, when entering the body through air, food or blood (e.g. a wound), mostly settles in the lungs, liver and bones with only about 10% going to other organs, and remains there for decades.