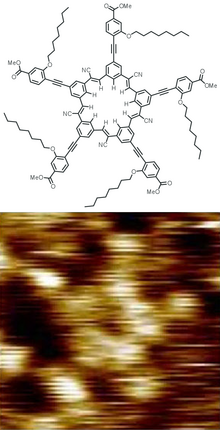
Cyanostar
A cyanostar (pentacyanopentabenzo[25]annulene) is a shape-persistent macrocycle that binds anions.
[1][2] The cyanostar structure is synthesized in a one-pot process among five equivalents of a benzaldehyde bearing a meta-cyanomethyl substituent.
A series of Knoevenagel condensation reactions catalyzed by various bases stitches them together to make the C5-symmetric structure.
The increased binding arises from the formation of a 2:1 complex, with two cyanostars sandwiching the anion on each side.
[3] An extended version of this structural pattern is a 4:3 alternating stack of cyanostar molecules complexing a hydrogen-bonded chain of dihydrogen phosphate units.