Rotaxane
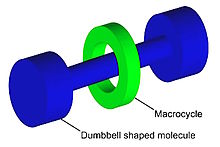
A rotaxane (from Latin rota 'wheel' and axis 'axle') is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation).
The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probability that if two halves of a dumbbell-shaped molecule were reacted in the presence of a macrocycle that some small percentage would connect through the ring.
[4][5] Recently, Leigh and co-workers described a new pathway to mechanically interlocked architectures involving a transition-metal center that can catalyse a reaction through the cavity of a macrocycle.
This dynamic complex or pseudorotaxane is then converted to the rotaxane by reacting the ends of the threaded guest with large groups, preventing disassociation.
[8] The clipping method is similar to the capping reaction except that in this case the dumbbell shaped molecule is complete and is bound to a partial macrocycle.
The partial macrocycle then undergoes a ring closing reaction around the dumbbell-shaped molecule, forming the rotaxane.
Leigh and co-workers recently began to explore a strategy in which template ions could also play an active role in promoting the crucial final covalent bond forming reaction that captures the interlocked structure (i.e., the metal has a dual function, acting as a template for entwining the precursors and catalyzing covalent bond formation between the reactants).
[22] The enhanced stability of rotaxane dyes is attributed to the insulating effect of the macrocycle, which is able to block interactions with other molecules.