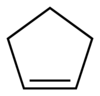
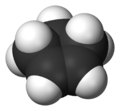
Cyclopentene
It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%.
Cyclopentene was first prepared by Carl Gärtner in 1893 from iodocyclopentane with potassium hydroxide.
[3] Cyclopentene is produced industrially in large amounts by steam cracking of naphtha.
The polymerization of cyclopentene by Ziegler-Natta catalysts yields 1,3-linkages, not the more typical 1,2-linked polymer.
[7] Palladium-catalyzed hydrocarboxylation of cyclopentene gives cyclopentanecarboxylic acid:[8] This article about a hydrocarbon is a stub.