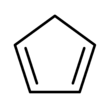
Cyclopentadiene
At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction.
The hydride shift is, however, sufficiently slow at 0 °C to allow alkylated derivatives to be manipulated selectively.
[8] The compound is unusually acidic (pKa = 16) for a hydrocarbon, a fact explained by the high stability of the aromatic cyclopentadienyl anion, C5H−5.
Metallocenes and related cyclopentadienyl derivatives have been heavily investigated and represent a cornerstone of organometallic chemistry owing to their high stability.
The first metallocene characterised, ferrocene, was prepared the way many other metallocenes are prepared by combining alkali metal derivatives of the form MC5H5 with dihalides of the transition metals:[12] As typical example, nickelocene forms upon treating nickel(II) chloride with sodium cyclopentadienide in THF.
Diels–Alder reaction with butadiene gives ethylidene norbornene, a comonomer in the production of EPDM rubbers.