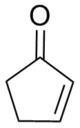
Cyclopentenone
Cyclopentenones are found in a large number of natural products, including jasmone, the aflatoxins, and several prostaglandins.
One of the routes involves elimination of α-bromo-cyclopentanone using lithium carbonate[2] and Claisen condensation-decarboxylation-isomerization cascades of unsaturated diesters as shown below.
[4] As a functional group, the synthesis of 2-cyclopentenones is accomplished in a variety of other ways, including the Nazarov cyclization reaction from divinyl ketones, Saegusa–Ito oxidation from cyclopentanones, ring-closing metathesis from the corresponding dienes, oxidation of the corresponding cyclic allylic alcohols, and the Pauson–Khand reaction from alkenes, alkynes, and carbon monoxide.
In one example, a Danishefsky-type diene is reacted with a cyclopentenone to yield a fused tricyclic system en route to the synthesis of coriolin.
[6] It has been isolated from pressure-cooked pork liver by simultaneous steam distillation and continuous solvent extraction.