Glucose
[6][8] The naturally occurring form is d-glucose, while its stereoisomer l-glucose is produced synthetically in comparatively small amounts and is less biologically active.
[12][13] Glucose was discovered in grapes by another German chemist – Johann Tobias Lowitz – in 1792, and distinguished as being different from cane sugar (sucrose).
Friedrich August Kekulé proposed the term dextrose (from the Latin dexter, meaning "right"), because in aqueous solution of glucose, the plane of linearly polarized light is turned to the right.
This understanding occurred largely as a result of the investigations of Emil Fischer, a German chemist who received the 1902 Nobel Prize in Chemistry for his findings.
[17] Between 1891 and 1894, Fischer established the stereochemical configuration of all the known sugars and correctly predicted the possible isomers, applying Van 't Hoff equation of asymmetrical carbon atoms.
[18] Hans von Euler-Chelpin was awarded the Nobel Prize in Chemistry along with Arthur Harden in 1929 for their "research on the fermentation of sugar and their share of enzymes in this process".
[29][30][31][32][33] The term dextrose is often used in a clinical (related to patient's health status) or nutritional context (related to dietary intake, such as food labels or dietary guidelines), while "glucose" is used in a biological or physiological context (chemical processes and molecular interactions),[34][35][36][37] but both terms refer to the same molecule, specifically D-glucose.
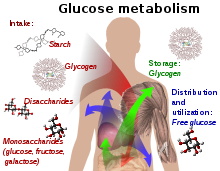
[55] Glucose is a building block of the disaccharides lactose and sucrose (cane or beet sugar), of oligosaccharides such as raffinose and of polysaccharides such as starch, amylopectin, glycogen, and cellulose.
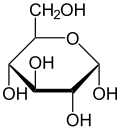
In its open-chain form, the glucose molecule has an open (as opposed to cyclic) unbranched backbone of six carbon atoms, where C-1 is part of an aldehyde group H(C=O)−.
In solutions, the open-chain form of glucose (either "D-" or "L-") exists in equilibrium with several cyclic isomers, each containing a ring of carbons closed by one oxygen atom.
The reaction between C-1 and C-5 yields a six-membered heterocyclic system called a pyranose, which is a monosaccharide sugar (hence "-ose") containing a derivatised pyran skeleton.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane.
In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation.
The effect is due to the chirality of the molecules, and indeed the mirror-image isomer, l-(−)-glucose, is levorotatory (rotates polarized light counterclockwise) by the same amount.
[65][66] Another hypothesis is that glucose, being the only d-aldohexose that has all five hydroxy substituents in the equatorial position in the form of β-d-glucose, is more readily accessible to chemical reactions,[67]: 194, 199 for example, for esterification[68]: 363 or acetal formation.
[72] Many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids.
In humans, the breakdown of glucose-containing polysaccharides happens in part already during chewing by means of amylase, which is contained in saliva, as well as by maltase, lactase, and sucrase on the brush border of the small intestine.
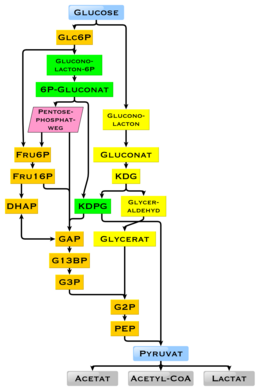
If there is not enough oxygen available for this, the glucose degradation in animals occurs anaerobic to lactate via lactic acid fermentation and releases much less energy.
[§ 1] Tumor cells often grow comparatively quickly and consume an above-average amount of glucose by glycolysis,[102] which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen.
[108] The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage.
[89][71] In ruminants, the blood glucose concentration is lower (60 mg/dL in cattle and 40 mg/dL in sheep), because the carbohydrates are converted more by their gut microbiota into short-chain fatty acids.
Some of these polymers (starch or glycogen) serve as energy stores, while others (cellulose and chitin, which is made from a derivative of glucose) have structural roles.
[127] Finally, glucose is used as a building block in the glycosylation of proteins to glycoproteins, glycolipids, peptidoglycans, glycosides and other substances (catalyzed by glycosyltransferases) and can be cleaved from them by glycosidases.
Each of these situations can be caused by persistently high elevations of blood glucose levels, through pancreatic burnout and insulin resistance.
Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose).
Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree Wollemia nobilis in Rome,[131] the roots of Ilex asprella plants in China,[132] and straws from rice in California.
[148][additional citation(s) needed] Potent, bioactive natural products like triptolide that inhibit mammalian transcription via inhibition of the XPB subunit of the general transcription factor TFIIH has been recently reported as a glucose conjugate for targeting hypoxic cancer cells with increased glucose transporter expression.
[149] Recently, glucose has been gaining commercial use as a key component of "kits" containing lactic acid and insulin intended to induce hypoglycemia and hyperlactatemia to combat different cancers and infections.
[157] The test-strip method employs the above-mentioned enzymatic conversion of glucose to gluconic acid to form hydrogen peroxide.
In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups of sugars, there are also other probe concepts classified by functional mechanisms which use selective glucose-binding proteins (e.g. concanavalin A) as a receptor.