Diethylstilbestrol
[7] Compared to estradiol, DES has greatly improved bioavailability when taken by mouth, is more resistant to metabolism, and shows relatively increased effects in certain parts of the body like the liver and uterus.
[10][5] The United States Food and Drug Administration subsequently withdrew approval of DES as a treatment for pregnant women.
[10][5] Follow-up studies have indicated that DES also has the potential to cause a variety of significant adverse medical complications during the lifetimes of those exposed including infertility.
[36][38] Oral DES at 0.25 to 0.5 mg/day is effective in the treatment of hot flashes in men undergoing androgen deprivation therapy for prostate cancer.
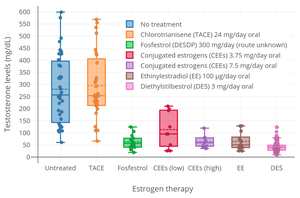
[40][41][42][43] At more than 1 mg/day, DES is associated with high rates of side effects including nausea, vomiting, abdominal discomfort, headache, and bloating (incidence of 15–50%).
[52] In men treated with it for prostate cancer, DES has been found to produce high rates of gynecomastia (breast development) of 41 to 77%.
[53] In studies of DES as a form of high-dose estrogen therapy for those with prostate cancer, it has been associated with considerable cardiovascular morbidity and mortality.
[64] In addition to the nuclear ERs, DES is an agonist of the G protein-coupled estrogen receptor (GPER), albeit with relatively low affinity (~1,000 nM).
[83] However, the addition of an "extremely low" dosage of 0.1 mg/day DES to cyproterone acetate has been found to result in a synergistic antigonadotropic effect and to suppress testosterone levels into the castrate range in men.
[102] In addition to the ERs, an in vitro study found that DES also possesses activity, albeit relatively weak, at a variety of other steroid hormone receptors.
[115][116][114][113] As shown by X-ray crystallography, the molecular dimensions of DES are almost identical to those of estradiol, particularly in regards to the distance between the terminal hydroxyl groups.
[113] DES was first synthesized in early 1938 by Leon Golberg, then a graduate student of Sir Robert Robinson at the Dyson Perrins Laboratory at the University of Oxford.
Golberg's research was based on work by Wilfrid Lawson at the Courtauld Institute of Biochemistry, (led by Sir Edward Charles Dodds at Middlesex Hospital Medical School now part of University College London).
[120][121] In 1941, Charles Huggins and Clarence Hodges at the University of Chicago found estradiol benzoate and DES to be the first effective drugs for the treatment of metastatic prostate cancer.
In 1971, a report published in the New England Journal of Medicine showed a probable link between DES and vaginal clear cell adenocarcinoma in girls and young women who had been exposed to this drug in utero.
[130] The number of persons exposed to DES during pregnancy or in utero during the period of 1940 to 1971 is unknown, but may be as high as 2 million in the United States.
[134][135][136] In 1973, in an attempt to restrict off-label use of DES as a postcoital contraceptive (which had become prevalent at many university health services following publication of an influential study in 1971 in JAMA) to emergency situations such as rape, an FDA Drug Bulletin was sent to all U.S. physicians and pharmacists that said the FDA had approved, under restricted conditions, postcoital contraceptive use of DES.
[140] In 1978, the FDA removed postpartum lactation suppression to prevent breast engorgement from their approved indications for DES and other estrogens.
[142] Proceeding the completion of the trial, it was understood that rats of older age who were injected with DES experienced delay in sertoli cell maturation, underdeveloped epididymides, and drastic decrease in weight compared to its counterparts.
In 1947, DES finally gained FDA approval for prescription to pregnant women who had diabetes as a method of preventing miscarriages.
[148] Medical Ethics in regard to the approval and use of Diethylstilbestrol have been dismissed because of the actions of the FDA and pharmaceutical companies that were making DES at the time of its use.
Nancy Langston, the author of The Retreat from Precaution: Regulating Diethylstilbestrol (DES), Endocrine Disruptors, and Environmental Health, states that "Frailey persuaded fifty-four doctors from around the country to write to the FDA, describing their clinical experiences with a total of more than five thousand patients.
The act of distributing potentially dangerous medicine to patients regardless of the effect and harm it may do solely for monetary gain is unethical.
[citation needed] In the 1970s, the negative publicity surrounding the discovery of DES's long-term effects resulted in a huge wave of lawsuits in the United States against its manufacturers.
[citation needed] Eli Lilly, a pharmaceutical company manufacturing DES, and the University of Chicago, had an action filed against them in regard to clinical trials from the 1950s.
In 2013, the Fecho sisters who initiated the breast cancer/DES link litigation agreed to an undisclosed settlement amount on the second day of trial.
[152] The advocacy group DES Action USA helped provide information and support for DES-exposed persons engaged in lawsuits.
[155] James Herriot describes a case regarding treating a small dog's testicular Sertoli cell tumor in his 1974 book All Things Bright and Beautiful.
"[citation needed] DES has been very successful in treating female canine incontinence stemming from poor sphincter control.
[citation needed] The greatest usage of DES was in the livestock industry, used to improve feed conversion in beef and poultry.