DRIP-seq
[1] DRIP-seq utilizes a sequence-independent but structure-specific antibody for DNA-RNA immunoprecipitation (DRIP) to capture R-loops for massively parallel DNA sequencing.
[1] An R-loop is a three-stranded nucleic acid structure, which consists of a DNA-RNA hybrid duplex and a displaced single stranded DNA (ssDNA).
[2] R-loops are predominantly formed in cytosine-rich genomic regions during transcription[2] and are known to be involved with gene expression and immunoglobulin class switching.
[1][3] Under abnormal conditions, namely elevated production of DNA-RNA hybrids, R-loops can cause genome instability by exposing single-stranded DNA to endogenous damages exerted by the action of enzymes such as AID and APOBEC, or overexposure to chemically reactive species.
[5] However, upon the arrival of massive parallel sequencing technologies and thereafter derivatives like DRIP-seq, the possibility to investigate entire genomes for R-loops has opened up.
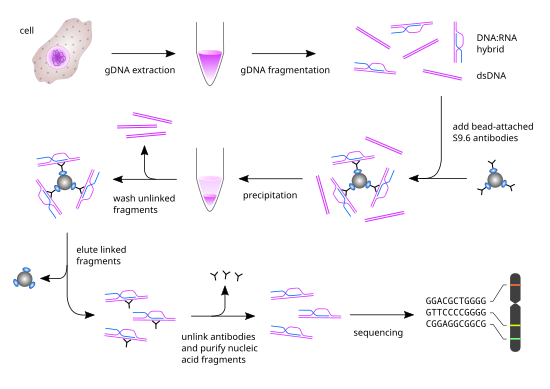
DRIP-seq relies on the high specificity and affinity of the S9.6 monoclonal antibody (mAb) towards DNA-RNA hybrids of various lengths.
[3] These types of studies provide information about the roles of the mutated gene in suppressing DNA-RNA formation and potentially about the significance of R-loops in genome instability.
DRIP-seq was later used to profile R-loop formation at transcription start and termination sites in human pluripotent Ntera2 cells.
This step is unique for the DRIP-seq protocol, since it entirely relies on the high specificity and affinity of the S9.6 mAb for DNA-RNA hybrids.
To remove the antibody bound to the nucleic acid hybrids, proteinase K treatment is performed followed by phenol-chloroform extraction and ethanol precipitation.