Decay scheme
These relations can be quite complicated; a simple case is shown here: the decay scheme of the radioactive cobalt isotope cobalt-60.
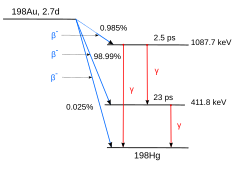
A somewhat more complicated scheme is shown here: the decay of the nuclide 198Au [4] which can be produced by irradiating natural gold in a nuclear reactor.
It is the decay of the element Polonium[6] discovered by Marie Curie, with mass number 210.
The isotope 210Po is the penultimate member of the uranium-radium-decay series; it decays into a stable lead-isotope with a half-life of 138 days.
Alpha- beta- and gamma rays can only be emitted if the conservation laws (energy, angular momentum, parity) are obeyed.