Demethylation
These enzymes oxidize N-methyl groups, which occur in histones, in lysine derivatives, and in some forms of DNA.
[6] These reactions, which proceed via hydroxylation, exploit the slightly weakened C-H bonds of methylamines and methyl ethers.
[13][14] Quantitative analysis for aromatic methyl ethers can be performed by argentometric determination of the N-methylpyridinium chloride formed.
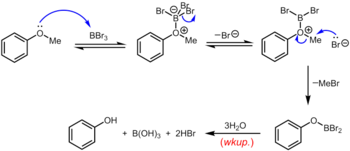
[18] Boron tribromide, which can be used at room temperature or below, is a more specialized reagent for the demethylation of aryl methyl ethers.
Rupture of the ether linkage occurs through the subsequent nucleophilic attack on the oxonium species by Br– to yield an aryloxydibromoborane and methyl bromide.
Highly specialized demethylations are abundant, such as the Krapcho decarboxylation: A mixture of anethole, KOH, and alcohol was heated in an autoclave.
N-demethylation of 3° amines is by the von Braun reaction, which uses BrCN as the reagent to give the corresponding nor- derivatives.
A modern variation of the von Braun reaction was developed, where BrCN was superseded by ethyl chloroformate.