Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair.
All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles.
In general, in a group across the periodic table, the more basic the ion (the higher the pKa of the conjugate acid) the more reactive it is as a nucleophile.
This treatment results in the following values for typical nucleophilic anions: acetate 2.7, chloride 3.0, azide 4.0, hydroxide 4.2, aniline 4.5, iodide 5.0, and thiosulfate 6.4.
The equation predicts that, in a nucleophilic displacement on benzyl chloride, the azide anion reacts 3000 times faster than water.
The Ritchie equation, derived in 1972, is another free-energy relationship:[6][7][8] where N+ is the nucleophile dependent parameter and k0 the reaction rate constant for water.
The equation states that two nucleophiles react with the same relative reactivity regardless of the nature of the electrophile, which is in violation of the reactivity–selectivity principle.
Many of the constants have been derived from reaction of so-called benzhydrylium ions as the electrophiles:[10] and a diverse collection of π-nucleophiles: Typical E values are +6.2 for R = chlorine, +5.90 for R = hydrogen, 0 for R = methoxy and −7.02 for R = dimethylamine.
This equation can be rewritten in several ways: Examples of nucleophiles are anions such as Cl−, or a compound with a lone pair of electrons such as NH3 (ammonia) and PR3.
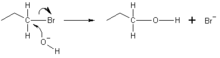
[citation needed] In the example below, the oxygen of the hydroxide ion donates an electron pair to form a new chemical bond with the carbon at the end of the bromopropane molecule.
Nitrogen nucleophiles include ammonia, azide, amines, nitrites, hydroxylamine, hydrazine, carbazide, phenylhydrazine, semicarbazide, and amide.
are most commonly cationic and electrophilic (Lewis acidic) in nature, certain metal centers (particularly ones in a low oxidation state and/or carrying a negative charge) are among the strongest recorded nucleophiles and are sometimes referred to as "supernucleophiles."