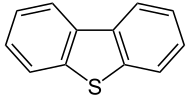
Dibenzothiophene
This tricyclic heterocycle, and especially its disubstituted derivative 4,6-dimethyldibenzothiophene are problematic impurities in petroleum.
[1] Dibenzothiophene is prepared by the reaction of biphenyl with sulfur dichloride in the presence of aluminium chloride.
[2] Reduction with lithium results in scission of one C-S bond.
[3] Dibenzothiophene is electron-rich, and naturally undergoes aromatic substitution para to the sulfide.
Oxidation to the sulfoxide or sulfone leaves the compound electron poor, and substitution occurs at the meta position instead.