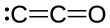Dicarbon monoxide
It is a linear molecule that, because of its simplicity, is of interest in a variety of areas.
It is, however, so extremely reactive that it is not encountered in everyday life.
[1] Dicarbon monoxide is a product of the photolysis of carbon suboxide:[2][3] It is stable enough to observe reactions with NO and NO2.
[4] Called ketenylidene in organometallic chemistry, it is a ligand observed in metal carbonyl clusters, e.g. [OC2Co3(CO)9]+.
Ketenylidenes are proposed as intermediates in the chain growth mechanism of the Fischer-Tropsch Process, which converts carbon monoxide and hydrogen to hydrocarbon fuels.