Diffusion
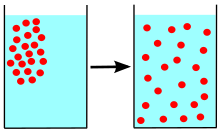
A distinguishing feature of diffusion is that it depends on particle random walk, and results in mixing or mass transport without requiring directed bulk motion.
When talking about the extent of diffusion, two length scales are used in two different scenarios: "Bulk flow" is the movement/flow of an entire body due to a pressure gradient (for example, water coming out of a tap).
In molecular diffusion, the moving molecules in a gas, liquid, or solid are self-propelled by kinetic energy.
[5] The concept of diffusion is typically applied to any subject matter involving random walks in ensembles of individuals.
In chemistry and materials science, diffusion also refers to the movement of fluid molecules in porous solids.
[7][8] Diffusion of the elements is critical in understanding the surface composition of degenerate white dwarf stars and their evolution over time.
For example, Pliny the Elder had previously described the cementation process, which produces steel from the element iron (Fe) through carbon diffusion.
He studied diffusion in gases, and the main phenomenon was described by him in 1831–1833:[10] "...gases of different nature, when brought into contact, do not arrange themselves according to their density, the heaviest undermost, and the lighter uppermost, but they spontaneously diffuse, mutually and equally, through each other, and so remain in the intimate state of mixture for any length of time."
The measurements of Graham contributed to James Clerk Maxwell deriving, in 1867, the coefficient of diffusion for CO2 in the air.
He used Graham's research, stating his goal as "the development of a fundamental law, for the operation of diffusion in a single element of space".
Robert Boyle demonstrated diffusion in solids in the 17th century[11] by penetration of zinc into a copper coin.
William Chandler Roberts-Austen, the well-known British metallurgist and former assistant of Thomas Graham studied systematically solid state diffusion on the example of gold in lead in 1896. :[12] "... My long connection with Graham's researches made it almost a duty to attempt to extend his work on liquid diffusion to metals."
The modern atomistic theory of diffusion and Brownian motion was developed by Albert Einstein, Marian Smoluchowski and Jean-Baptiste Perrin.
He concluded, the diffusion process in condensed matter is an ensemble of elementary jumps and quasichemical interactions of particles and defects.
[12] Henry Eyring, with co-authors, applied his theory of absolute reaction rates to Frenkel's quasichemical model of diffusion.
In 1931, Lars Onsager[16] included the multicomponent transport processes in the general context of linear non-equilibrium thermodynamics.
The thermodynamic forces for the transport processes were introduced by Onsager as the space gradients of the derivatives of the entropy density
[15] The Einstein relation (kinetic theory) connects the diffusion coefficient and the mobility (the ratio of the particle's terminal drift velocity to an applied force).
[citation needed] The term "gram-ion" ("gram-particle") is used for a quantity of a substance that contains the Avogadro number of ions (particles).
In this notation, the Teorell formula for the flux has a very simple form[15] The standard derivation of the activity includes a normalization factor and for small concentrations
The alternative Langevin equation starts with Newton's second law of motion:[20] where Solving this equation, one obtained the time-dependent diffusion constant in the long-time limit and when the particle is significantly denser than the surrounding fluid,[20] where At long time scales, Einstein's result is recovered, but short time scales, the ballistic regime are also explained.
The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places.
In the Chapman–Enskog approximation, all the distribution functions are expressed through the densities of the conserved quantities:[13] The kinetic temperature T and pressure P are defined in 3D space as where
10 of the classical Chapman and Cowling book[13]) We can see that the dependence on T for the rigid spheres is the same as for the simple mean free path theory but for the power repulsion laws the exponent is different.
In applications to gas dynamics, the diffusion flux and the bulk flow should be joined in one system of transport equations.
Analytical and numerical models that solve the diffusion equation for different initial and boundary conditions have been popular for studying a wide variety of changes to the Earth's surface.
The movement of a substance within a mixture by "random walk" is governed by the kinetic energy within the system that can be affected by changes in concentration, pressure or temperature.
Thus, the study of the movement of a single atom becomes more subtle since particles at such small scales are described by probability amplitudes rather than deterministic measures of position and velocity.)
While Brownian motion of multi-molecular mesoscopic particles (like pollen grains studied by Brown) is observable under an optical microscope, molecular diffusion can only be probed in carefully controlled experimental conditions.
In contrast, heat conduction through solid media is an everyday occurrence (for example, a metal spoon partly immersed in a hot liquid).