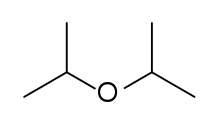
Diisopropyl ether
It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents.
Diisopropyl ether is used as a specialized solvent to remove or extract polar organic compounds from aqueous solutions, e.g. phenols, ethanol, acetic acid.
[4][5] Diisopropyl ether is used for converting bromoboranes, which are thermally labile, into isopropoxy derivatives.
This reaction proceeds more easily than for ethyl ether due to the increased lability of the C-H bond adjacent to oxygen.
[8] Peroxides may be removed by shaking the ether with an aqueous solution of iron(II) sulfate or sodium metabisulfite.