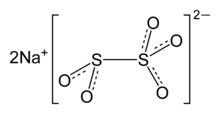
Sodium metabisulfite
[3] When conducted in warm water, Na2SO3 initially precipitates as a yellow solid.
With more SO2, the solid dissolves to give the disulfite, which crystallises upon cooling.
[5] Upon dissolution in water, bisulfite is generated: Sodium and potassium metabisulfite have many major and niche uses.
Some people who are sulfite sensitive may experience an allergic reaction to sodium meta bisulfite, sometimes severe, resulting in labeling requirements for food safety.
[20] In 2024, it was named ‘allergen of the year 2024’ by the American Contact Dermatitis Society.