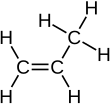
Propylene
[4] Propylene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust.
[5] It was discovered in 1850 by A. W. von Hoffmann's student Captain (later Major General[6]) John Williams Reynolds as the only gaseous product of thermal decomposition of amyl alcohol to react with chlorine and bromine.
Cracking propane yields a mixture of ethylene, propylene, methane, hydrogen gas, and other related compounds.
[8] Propylene can be separated by fractional distillation from the hydrocarbon mixtures obtained from cracking and other refining processes; refinery-grade propene is about 50 to 70%.
Rhenium and molybdenum catalysts are used:[10] The technology is founded on an olefin metathesis reaction discovered at Phillips Petroleum Company.
A high severity FCC unit is usually fed with gas oils (paraffins) and residues, and produces about 20–25% (by mass) of propene on feedstock together with greater volumes of motor gasoline and distillate byproducts.
Propene production has remained static at around 35 million tonnes (Europe and North America only) from 2000 to 2008, but it has been increasing in East Asia, most notably Singapore and China.
This poses several advantages, as this reaction mechanism can occur at lower temperatures than conventional dehydrogenation, and may not be equilibrium-limited because oxygen is used to combust the hydrogen by-product.
In industry and workshops, propylene is used as an alternative fuel to acetylene in Oxy-fuel welding and cutting, brazing and heating of metal for the purpose of bending.
Propylene resembles other alkenes in that it undergoes electrophilic addition reactions relatively easily at room temperature.
Propene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust.
[9] In the United States and some European countries a threshold limit value of 500 parts per million (ppm) was established for occupational (8-hour time-weighted average) exposure.
[28] On September 30, 2013, NASA announced the detection of small amounts of naturally occurring propene in the atmosphere of Titan using infrared spectroscopy.
[29][30][31] The detection was made by a team led by NASA GSFC scientist Conor Nixon using data from the CIRS instrument [32][33] on the Cassini orbiter spacecraft, part of the Cassini-Huygens mission.
Its confirmation solved a 32-year old mystery by filling a predicted gap in Titan's detected hydrocarbons, adding the C3H6 species (propene) to the already-detected C3H4 (propyne) and C3H8 (propane).