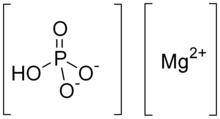
Dimagnesium phosphate
Dimagnesium phosphate is a compound with formula MgHPO4.
It is a Mg2+ salt of monohydrogen phosphate.
[1] It can be formed by reaction of stoichiometric quantities of magnesium oxide with phosphoric acid.
Dissolving monomagnesium phosphate in water, forms phosphoric acid and depositing a solid precipitate of dimagnesium phosphate trihydrate: The compound is used as a nutritional supplement, especially for infants and athletes.
[2] This inorganic compound–related article is a stub.