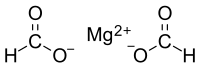Magnesium formate
It consists of a magnesium cation and formate anion.
It can be prepared by reacting magnesium oxide with formic acid.
The dihydrate is formed when crystallizing from the solution.
The dihydrate dehydrates at 105 °C to form anhydrate, then decomposes at 500 °C to produce magnesium oxide.
[1] Magnesium formate can be used for organic syntheses.