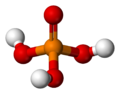
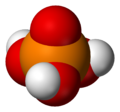
Phosphoric acid
The hydrogen fluoride (HF) gas is streamed into a wet (water) scrubber producing hydrofluoric acid.
The phosphoric acid from both processes may be further purified by removing compounds of arsenic and other potentially toxic impurities.
To produce food-grade phosphoric acid, phosphate ore is first reduced with coke in an electric arc furnace, to give elemental phosphorus.
[22] The thermal process produces phosphoric acid with a very high concentration of P2O5 (about 85%) and a low level of impurities.
A common purification methods is liquid-liquid extraction, which involves the separation of phosphoric acids from water and other impurities using organic solvents, such as tributyl phosphate (TBP), methyl isobutyl ketone (MIBK), or n-octanol.
Nanofiltration has been shown to significantly reduce the concentrations of various impurities, including cadmium, aluminum, iron, and rare earth elements.
The laboratory and industrial pilot scale results showed that this process allows the production of food-grade phosphoric acid.
The process begins with the slow cooling of the heat transfer medium below the freezing point of the stagnant melt.
In a subsequent step, the plates are heated again to liquify the crystals and the purified phosphoric acid drained into the product vessel.
For this reason phosphoric acid is rarely sold above 85%, as beyond this adding or removing small amounts of moisture risks the entire mass freezing solid, which would be a major problem on a large scale.
Concentrated phosphoric acid tends to supercool before crystallization occurs, and may be relatively resistant to crystallisation even when stored below the freezing point.
[31] Food-grade phosphoric acid (additive E338[32]) is used to acidify foods and beverages such as various colas and jams, providing a tangy or sour taste.
[42] A link has been shown between long-term regular cola intake and osteoporosis in later middle age in women (but not men).