Dimethylformamide
Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine.
As for most amides, the spectroscopic evidence indicates partial double bond character for the C−N and C−O bonds.Thus, the infrared spectrum shows a C=O stretching frequency at only 1675 cm−1, whereas a ketone would absorb near 1700 cm−1.
[7] The ambient temperature 1H NMR spectrum shows two methyl signals, indicative of hindered rotation about the (O)C−N bond.
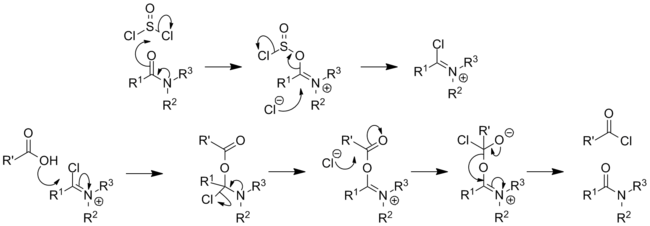
[13][14] The process involves initial conversion of DMF to a chloroiminium ion, [(CH3)2N=CH(Cl)]+, known as a Vilsmeier reagent,[15] which attacks arenes.
[17] Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by C-B plots.
[18][19] DMF was first obtained in 1893 by the French chemist Albert Verley (1867–1959), by distilling a mixture of dimethylamine hydrochloride and potassium formate.
It is also used as a solvent in peptide coupling for pharmaceuticals, in the development and production of pesticides, and in the manufacture of adhesives, synthetic leathers, fibers, films, and surface coatings.
Dimethylformamide vapor exposure has shown reduced alcohol tolerance and skin irritation in some cases.