Distillation
[2] Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity.
[3] The key issue is that distillation operates based on phase changes, and this separation mechanism requires vast energy inputs.
In either method, the separation process of distillation exploits the differences in the relative volatility of the component substances of the heated mixture.
The tablets provided textual evidence that an early, primitive form of distillation was known to the Babylonians of ancient Mesopotamia.
[9]According to Dutch chemical historian Robert J. Forbes, the word distillare (to drip off) when used by the Romans, e.g. Seneca and Pliny the Elder, was "never used in our sense".
[12] Early evidence of distillation has been found related to alchemists working in Alexandria in Roman Egypt in the 1st century CE.
Distillation was practiced in the ancient Indian subcontinent, which is evident from baked clay retorts and receivers found at Taxila, Shaikhan Dheri, and Charsadda in Pakistan and Rang Mahal in India dating to the early centuries of the Common Era.
[22] These "Gandhara stills" were only capable of producing very weak liquor, as there was no efficient means of collecting the vapors at low heat.
The fractional distillation of organic substances plays an important role in the works attributed to Jābir, such as in the Kitāb al-Sabʿīn ('The Book of Seventy'), translated into Latin by Gerard of Cremona (c. 1114–1187) under the title Liber de septuaginta.
[25] The Jabirian experiments with fractional distillation of animal and vegetable substances, and to a lesser degree also of mineral substances, is the main topic of the De anima in arte alkimiae, an originally Arabic work falsely attributed to Avicenna that was translated into Latin and would go on to form the most important alchemical source for Roger Bacon (c. 1220–1292).
[24] A still was found in an archaeological site in Qinglong, Hebei province, China, dating back to the 12th century.
[31] In 1651, John French published The Art of Distillation,[32] the first major English compendium on the practice, but it has been claimed[33] that much of it derives from Brunschwig's work.
[34] These alembics often featured a cooling system around the beak, using cold water, for instance, which made the condensation of alcohol more efficient.
[16]: 323 In 1877, Ernest Solvay was granted a U.S. Patent for a tray column for ammonia distillation,[38] and the same and subsequent years saw developments in this theme for oils and spirits.
The first industrial plant in the United States to use distillation as a means of ocean desalination opened in Freeport, Texas in 1961 with the hope of bringing water security to the region.
Although there are computational methods that can be used to estimate the behavior of a mixture of arbitrary components, the only way to obtain accurate vapor–liquid equilibrium data is by measurement.
A completely sealed distillation apparatus could experience extreme and rapidly varying internal pressure, which could cause it to burst open at the joints.
If the substances involved are air- or moisture-sensitive, the connection to the atmosphere can be made through one or more drying tubes packed with materials that scavenge the undesired air components, or through bubblers that provide a movable liquid barrier.
Finally, the entry of undesired air components can be prevented by pumping a low but steady flow of suitable inert gas, like nitrogen, into the apparatus.
The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate and magnetic stirrer 13 via a silicone oil bath (orange, 14).
The vapor flows through a short Vigreux column 3, then through a Liebig condenser 5, is cooled by water (blue) that circulates through ports 6 and 7.
[42] In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionating column; theoretical plate is thus a concept rather than an accurate description.
To do this a "cow" or "pig" adaptor can be added to the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used.
The Perkin triangle has means via a series of glass or Teflon taps to allows fractions to be isolated from the rest of the still, without the main body of the distillation being removed from either the vacuum or heat source, and thus can remain in a state of reflux.
When zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming.
The process may be iterated many times by moving (without turnover) the received condensate to the bottom part of the container on the place of refined matter.
At an azeotrope, the solution contains the given component in the same proportion as the vapor, so that evaporation does not change the purity, and distillation does not result in separation.
Chemical engineers must choose what combination of reflux rate and number of plates is both economically and physically feasible for the products purified in the distillation column.
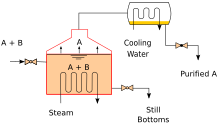
[54] The goal of multi-effect distillation is to increase the energy efficiency of the process, for use in desalination, or in some cases one stage in the production of ultrapure water.
Components other than ethanol, including water, esters, and other alcohols, are collected in the condensate, which account for the flavor of the beverage.
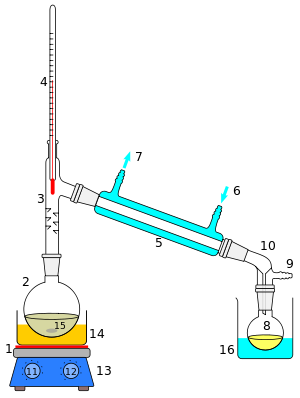
1: The heat source to boil the mixture
2: round-bottom flask containing the mixture to be boiled
3: the head of the still
4: mixture boiling-point thermometer
5: the condenser of the still
6: the cooling-water inlet of the condenser
7: the cooling-water outlet of the condenser
8: the distillate-receiving flask
9: vacuum pump and gas inlet
10: the receiver of the still
11: the heat control for heating the mixture
12: stirring mechanism speed control
13: stirring mechanism and heating plate
14: heating bath (oil/sand) for the flask
15: the stirring mechanism (not shown, e.g. boiling chips or mechanical stirring machine)
16: the distillate-cooling water bath. [ 1 ] : 141–143








- Stirrer bar/anti-bumping granules
- Still pot
- Fractionating column
- Thermometer/Boiling point temperature
- Teflon tap 1
- Cold finger
- Cooling water out
- Cooling water in
- Teflon tap 2
- Vacuum/gas inlet
- Teflon tap 3
- Still receiver
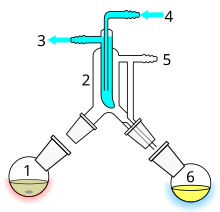
- Still pot with stirrer bar/anti-bumping granules
- Cold finger – bent to direct condensate
- Cooling water out
- cooling water in
- Vacuum/gas inlet
- Distillate flask/distillate.