Sublimatory
A typical sublimation apparatus separates a mix of appropriate solid materials in a vessel in which it applies heat under a controllable atmosphere (air, vacuum or inert gas).
If the operation is a batch process, then the sublimed material can be collected from the cooled surface once heating ceases and the vacuum is released.
Among the advantages of applying the principle to certain materials are the comparatively low working temperatures, reduced exposure to gases such as oxygen that might harm certain products, and the ease with which it can be performed on extremely small quantities.
More sophisticated variants of sublimation apparatus include those that apply a temperature gradient so as to allow for controlled recrystallization of different fractions along the cold surface.
Thermodynamic processes follow a statistical distribution, and suitably designed apparatus exploit this principle with a gradient that will yield different purities in particular temperature zones along the collection surface.
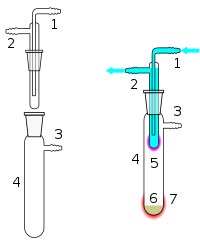
1 Cooling water in 2 Cooling water out 3 Vacuum/gas line 4 Sublimation chamber 5 Sublimed compound 6 Crude material 7 External heating

