Einstein coefficients
Throughout this article, "light" refers to any electromagnetic radiation, not necessarily in the visible spectrum.
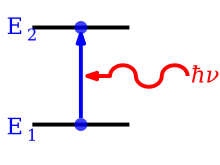
An absorption line is formed when an atom or molecule makes a transition from a lower, E1, to a higher discrete energy state, E2, with a photon being absorbed in the process.
[2][3][4][5][6][7] An atomic spectral line refers to emission and absorption events in a gas in which
ε dt dV dΩ is then the energy emitted by a volume element
For thermodynamics and for the application of Kirchhoff's law, it is necessary that the total absorption be expressed as the algebraic sum of two components, described respectively by
To be accurate, the above equations need to be multiplied by the (normalized) spectral line shape, in which case the units will change to include a 1/Hz term.
, the Einstein coefficients, and the spectral energy density provide sufficient information to determine the absorption and emission rates.
For local thermodynamic equilibrium, the radiation field does not have to be a black-body field, but the rate of interatomic collisions must vastly exceed the rates of absorption and emission of quanta of light, so that the interatomic collisions entirely dominate the distribution of states of atomic excitation.
Circumstances occur in which local thermodynamic equilibrium does not prevail, because the strong radiative effects overwhelm the tendency to the Maxwell–Boltzmann distribution of molecular velocities.
In the upper atmosphere of the Earth, at altitudes over 100 km, the rarity of intermolecular collisions is decisive.
In the cases of thermodynamic equilibrium and of local thermodynamic equilibrium, the number densities of the atoms, both excited and unexcited, may be calculated from the Maxwell–Boltzmann distribution, but for other cases, (e.g. lasers) the calculation is more complicated.
In 1916, Albert Einstein proposed that there are three processes occurring in the formation of an atomic spectral line.
[3][14][15][16] Paul Dirac derived the coefficients in a 1927 paper titled "The Quantum Theory of the Emission and Absorption of Radiation".
The process is described by the Einstein coefficient A21 (s−1), which gives the probability per unit time that an electron in state 2 with energy
Due to the energy-time uncertainty principle, the transition actually produces photons within a narrow range of frequencies called the spectral linewidth.
Stimulated emission (also known as induced emission) is the process by which an electron is induced to jump from a higher energy level to a lower one by the presence of electromagnetic radiation at (or near) the frequency of the transition.
The change in the number density of atoms in state 1 per unit time due to induced emission will be
denotes the spectral energy density of the isotropic radiation field at the frequency of the transition (see Planck's law).
Absorption is the process by which a photon is absorbed by the atom, causing an electron to jump from a lower energy level to a higher one.
The change in the number density of atoms in state 1 per unit time due to absorption will be
Detailed balance (valid only at equilibrium) requires that the change in time of the number of atoms in level 1 due to the above three processes be zero:
where n is the total number density of the atomic species, excited and unexcited, k is the Boltzmann constant, T is the temperature,
Substituting these expressions into the equation of detailed balancing and remembering that E2 − E1 = hν yields
This allows all three Einstein coefficients to be expressed in terms of the single oscillator strength associated with the particular atomic spectral line:
The value of A and B coefficients can be calculated using quantum mechanics where dipole approximations in time dependent perturbation theory is used.
For B coefficient, straightforward application of dipole approximation in time dependent perturbation theory yields (in SI units):[30][29]
Note that the rate of transition formula depends on dipole moment operator.
For higher order approximations, it involves quadrupole moment and other similar terms.
[19] The formulas for B coefficients varies inversely to that of the energy distribution chosen, so that the transition rate is same regardless of convention.
Note that from time dependent perturbation theory application, the fact that only radiation whose