Electrochemical coloring of metals
[3][4] Anodic oxidation of aluminum, titanium, niobium, tantalum and stainless steel are also electrochemical colouring processes.
Apart from Leopoldo Nobili, who already in 1824 performed his first experiments related to the appearance of Nobili's rings, Leonhard Elsner, Alexander Watt, Antoine César Becquerel (1788–1878) and Rudolf Christian Böttger (1806–1881) also dealt with the electrochemical coloring of metals in that early period.
[9] We also know that George Richards Elkington (1801–1865), otherwise known for his patent for galvanic gilding and silver plating from 1840 patented at least one process of electrochemical coloring of metals (the American J.E .Stareck developed ten variants of his process around 1937).
[11] Around the same time, the first procedures for electrolytic browning of steel were developed (HL Hollis' patent USPT 621,084 from 1899 was the first attempt in this direction, but Becquerel reported on this already in 1861).
[12][13] While the aforementioned deal with this issue primarily for protection against corrosion, the English patent 106,774 from 1916 and the American patent by T. Rondelli and Q. Sestini USPT 1,386,076 from 1921 are also oriented towards the chemical coloring of steel, and iron as the goal of the procedure.
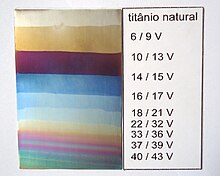
In the 1960s, procedures were developed for the anodic oxidation of titanium, a little later niobium and tantalum, and a little bit earlier stainless steel (circa 1957 patent US 2957812 A).
Several significant procedures were also developed in the former Soviet Union after the Second World War, the Ukrainian A.P.
Eitchis developed several complex, but also original procedures, which included the electrochemical coloring of metals (Kristalit, Iskrit, Sloit, Texturit - His work was strongly influenced by already mentioned J.E.Stareck).
[18] Nobili rings, Lismann green for copper and alloys, anodic oxidation of aluminum, magnesium, titanium, niobium, tantalum, tungsten, carbon steel and stainless steel, silver, copper and its alloys, tin, chromium and zinc.
[19] 39 gr of lead acetate 100 ml of distilled water cathode made of platinum or stainless steel (needle), anode nickel-plated or gold-plated copper or brass or polished steel, duration 10 s, distance between cathode and anode 3 mm .
[21] copper sulfate 75 gr/lit Sodium hydroxide 75 gr/lit lactic acid 126ml/lit copper anodes, 0.25/A per square foot, gives various colors on copper and alloys, depending on the duration of the process, a large number of variations on this process have been developed, the most famous is the American Elektrocolor process developed by J.E.Stareck, Russian literature mentions more than 10 variants [22][23][10] A 3% trisodium phosphate solution can be used as a simple electrolyte, a stainless steel cathode, an object as an anode.
Straw Yellow/10v - Magenta/29v - Blue/30v - Blue Green/45v - Bright Green/55v - Magenta Red/75v - Gray/110v It is mandatory to do this process with rubber gloves - potentially dangerous voltage.
55C, 0.5 - 1.5 V, 5 - 20 A /per square foot, nickel carbon anodes[27][28] sulfuric acid 250 ml/lit sodium bichromate 60 gr/lit water 1 lit 0.6 A/per square foot, 70 - 95 C, lead cathode, gives brown, blue, purple and green color depending on the duration of the process, there are many variants of this process.
[30] According to a Chinese patent, additionally objects can then be treated with a hot sodium waterglass solution (1 - 5%, 95-100 C, 3 - 10 min.).
[31] As hexavalent chromium compounds are prohibited for use in the EU based on ROHS regulations and are toxic and carcinogenic, solutions based on molybdate are proposed as a replacement (e.g. molybdate 30-100g/ boric acid 10-18 g/manganese sulfate 0.5 - 5 g/1 liter of water, 0.1 - 20 A/dm2, 0.1–15 minutes).