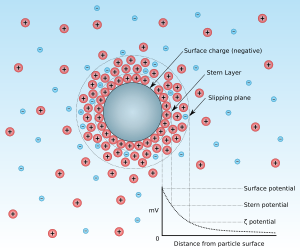
Zeta potential
In the colloidal chemistry literature, it is usually denoted using the Greek letter zeta (ζ), hence ζ-potential.
The magnitude of the zeta potential indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in a dispersion.
For molecules and particles that are small enough, a high zeta potential will confer stability, i.e., the solution or dispersion will resist aggregation.
[7] Zeta potential can also be used for the pKa estimation of complex polymers that is otherwise difficult to measure accurately using conventional methods.
Several sensors in the instrument monitor other factors, so the software attached is able to do calculations to find the zeta potential.
Electrophoresis is used for estimating zeta potential of particulates, whereas streaming potential/current is used for porous bodies and flat surfaces.
The frequency shift or phase shift of an incident laser beam caused by these moving particles is measured as the particle mobility, and this mobility is converted to the zeta potential by inputting the dispersant viscosity and dielectric permittivity, and the application of the Smoluchowski theories.
From the instrumental viewpoint, there are three different experimental techniques: microelectrophoresis, electrophoretic light scattering, and tunable resistive pulse sensing.
From the inverse translocation time versus voltage-dependent electrophoretic mobility, and thus zeta potentials are calculated.
The main advantage of the TRPS method is that it allows for simultaneous size and surface charge measurements on a particle-by-particle basis, enabling the analysis of a wide spectrum of synthetic and biological nano/microparticles and their mixtures.
In this case, the interfacial equilibrium between the surface and the bulk liquid would be maintained and zeta potential would be the same for all volume fractions of particles in the suspension.
Materials with an irregular shape, such as fibers or granular media, are mounted as a porous plug to provide a pore network, which serves as capillaries for the streaming potential measurement.
[20] There are two electroacoustic effects that are widely used for characterizing zeta potential: colloid vibration current and electric sonic amplitude.
[5] There are commercially available instruments that exploit these effects for measuring dynamic electrophoretic mobility, which depends on zeta potential.
Calculation of zeta potential from the dynamic electrophoretic mobility requires information on the densities for particles and liquid.
[citation needed] The most known and widely used theory for calculating zeta potential from experimental data is that developed by Marian Smoluchowski in 1903.
However, it has its limitations: The development of electrophoretic and electroacoustic theories with a wider range of validity was a purpose of many studies during the 20th century.
There are several analytical theories that incorporate surface conductivity and eliminate the restriction of the small Dukhin number for both the electrokinetic and electroacoustic applications.
, stem mostly from Soviet Ukrainian (Dukhin, Shilov, and others) and Australian (O'Brien, White, Hunter, and others) schools.
[25] Assuming a thin double layer, these theories would yield results that are very close to the numerical solution provided by O'Brien and White.