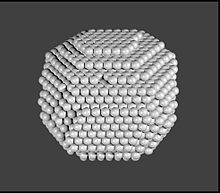
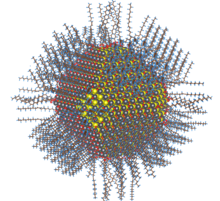
Nanoparticle
In general, the small size of nanoparticles leads to a lower concentration of point defects compared to their bulk counterparts,[7] but they do support a variety of dislocations that can be visualized using high-resolution electron microscopes.
[12][13] Non-spherical nanoparticles of gold (Au), silver (Ag), and platinum (Pt) due to their fascinating optical properties are finding diverse applications.
[14] The possibility of shifting the resonance wavelengths by tuning the particle geometry allows using them in the fields of molecular labeling, biomolecular assays, trace metal detection, or nanotechnical applications.
Anisotropic nanoparticles display a specific absorption behavior and stochastic particle orientation under unpolarized light, showing a distinct resonance mode for each excitable axis.
"[27][28][29] During the 1970s and 80s, when the first thorough fundamental studies with nanoparticles were underway in the United States by Granqvist and Buhrman[30] and Japan within an ERATO Project,[31] researchers used the term ultrafine particles.
However, during the 1990s, when the National Nanotechnology Initiative was launched in the United States, the term nanoparticle became more common, for example, see the same senior author's paper 20 years later addressing the same issue, lognormal distribution of sizes.
[citation needed] The breakdown of biopolymers into their nanoscale building blocks is considered a potential route to produce nanoparticles with enhanced biocompatibility and biodegradability.
[6] Suspensions of nanoparticles are possible since the interaction of the particle surface with the solvent is strong enough to overcome density differences, which otherwise usually result in a material either sinking or floating in a liquid.
[citation needed] The high surface area of a material in nanoparticle form allows heat, molecules, and ions to diffuse into or out of the particles at very large rates.
[77] The colloidal probe technique overcomes these issues by attaching a nanoparticle to the AFM tip, allowing control oversize, shape, and material.
For spherical polymer nanoparticles, glass transition temperature and crystallinity may affect deformation and change the elastic modulus when compared to the bulk material.
[86][87][88][93] There are several methods for creating nanoparticles, including gas condensation, attrition, chemical precipitation,[94] ion implantation, pyrolysis, hydrothermal synthesis, and biosynthesis.
[96][97][98] Biopolymers like cellulose, lignin, chitin, or starch may be broken down into their individual nanoscale building blocks, obtaining anisotropic fiber- or needle-like nanoparticles.
[citation needed] Another method to create nanoparticles is to turn a suitable precursor substance, such as a gas (e.g. methane) or aerosol, into solid particles by combustion or pyrolysis.
[100] Condensation of the supersaturated metal vapor results in creation of nanometer-size particles, which can be entrained in the inert gas stream and deposited on a substrate or studied in situ.
Formation of nanoparticles using the radiolysis method allows for tailoring of particle size and shape by adjusting precursor concentrations and gamma dose.
The size of the particles of the latter is adjusted by choosing the concentration of the reagents and the temperature of the solutions, and through the addition of suitable inert agents that affect the viscosity and diffusion rate of the liquid.
[107] The nanoparticles formed by this method are then separated from the solvent and soluble byproducts of the reaction by a combination of evaporation, sedimentation, centrifugation, washing, and filtration.
Even small quantities of dopants, such as organic dyes and rare earth metals, can be introduced in the reagent solutions end up uniformly dispersed in the final product.
[109][110] Ion implantation may be used to treat the surfaces of dielectric materials such as sapphire and silica to make composites with near-surface dispersions of metal or oxide nanoparticles.
[citation needed] Many properties of nanoparticles, notably stability, solubility, and chemical or biological activity, can be radically altered by coating them with various substances — a process called functionalization.
[112][113] By the immobilization of thiol groups on the surface of nanoparticles or by coating them with thiomers high (muco)adhesive and cellular uptake enhancing properties can be introduced.
Multivalent nanoparticles, bearing multiple targeting groups, can cluster receptors, which can activate cellular signaling pathways, and give stronger anchoring.
[citation needed] Uncontrolled agglomeration of powders due to attractive van der Waals forces can also give rise to microstructural heterogeneity.
Differential stresses that develop as a result of non-uniform drying shrinkage are directly related to the rate at which the solvent can be removed, and thus highly dependent upon the distribution of porosity.
purification) nature of the process and having enough time to form single crystal particles, however even their non-aggreated deposits have lognormal size distribution, which is typical with nanoparticles.
[31] However, even in this case, random residence times in the growth zone, due to the combination of drift and diffusion, result in a size distribution appearing lognormal.
Additionally, microscopy is based on single-particle measurements, meaning that large numbers of individual particles must be characterized to estimate their bulk properties.
[165] Clay nanoparticles, when incorporated into polymer matrices, increase reinforcement, leading to stronger plastics, verifiable by a higher glass transition temperature and other mechanical property tests.
Zinc oxide nanoparticles have been found to have superior UV blocking properties and are widely used in the preparation of sunscreen lotions,[171] being completely photostable[172] though toxic.