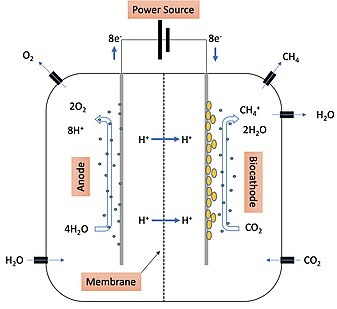
Electromethanogenesis
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide.
[1][4] Electrical current produced from renewable energy sources may, through electromethanogenesis, be converted into methane which may then be used as a biofuel.
[1][5][6] Abiogenic methane is produced on a smaller scale and the required chemical reactions do not necessitate organic materials.
[4][7] Researchers have found that the biogenic methane production process can be replicated in a laboratory environment through electromethanogenesis.
A biocathode is a cathode used in a microbial electrolysis cell during electromethanogenesis that utilizes microorganisms to catalyze the process of accepting electrons and protons from the anode.
Because the biocathode is so important in electron exchange and methane formation, its make-up can have a dramatic effect on the efficiency of the reaction.