Enediyne
The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule.
The resulting formation is a 1,4-benzenoid diradical fused to a ring composed of the leftover atoms from the original enediyne.
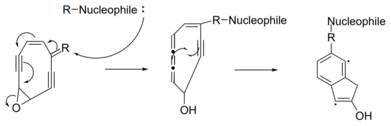
Some enediynes have an epoxide group attached to their ring, making Bergman cyclization unfavorable due to steric hindrance.
This mechanism requires the alkene of the enediyne to be part of a diene with a double bond in a variable group.
A nucleophile will attack the double bond in the variable region, causing a chain reaction of electron pushing.
[citation needed] The cyclization of the enediyne functional group creates a transient reactive 1,4-benzenoid diradical that acts as a nucleophile and attacks electrophiles in order to achieve a more stable form.
[5] The enediyne cores are derived from linear, probably polyketide, precursors that consist of seven or eight head-to-tail coupled acetate units.
These moieties can be either aromatic or sugars and define sequence specificity of DNA binding as well as the physical properties of the enediyne chromophores.
[5] Due to the cytotoxicity of the enediyne chromophores, their biosynthesis is tightly regulated, although the regulatory mechanisms are still largely unclear.
Notably, some microbes use CalC to sequester calicheamicin so that the reactive diradical abstracts hydrogens from a glycine inside of the protein instead of from DNA.
[13] The calicheamicins are notably similar in structure to the esperamicins.The esperamicins are a sub-family of enediynes that are considered among the most potent antitumor antibiotics discovered.
[17] Golfomycin A is a synthetic enediyne molecule designed in an attempt to create a more easily manufactured antitumor antibiotic.