Kedarcidin
Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism.
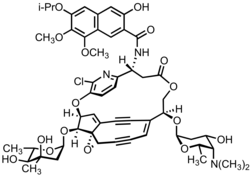
[1] Subsequent NMR, mass spectrometry, chemical degradation, and derivatization experiments enabled the isolation team to identify the key structural features of kedarcidin chromophore, including the enediyne bicyclic core, the ansa-bridging chloropyridyl ring, the mycarose and kedarosamine sugars, and the naphthoamide appendage.
In 1997, en route to the originally reported structure, researchers under the direction of Masahiro Hirama discovered that the spectroscopic data of the proposed chloroazatyrosyl (S)-α-amino acid derivative were not consistent with those of the degradation product characterized by Leet et al.
[3] Like other enediynes, kedarcidin chromophore comprises a core structure that forms destructive free radicals, as well as appendages that deliver this "warhead" to its DNA target.
14 kcal·mol−1) to the [6,5,5] tricycle formed upon Bergman cyclization–reduction, Hirama et al. note that the 5,9-fused enediyne core is susceptible to cycloaromatization–reduction in the absence of both thiol "activating agents" and (non-solvent) hydrogen donors.
[8] It is noteworthy that of the solvents examined, tetrahydrofuran—structurally homologous with deoxyribose—led to comparatively fast decomposition of the 5,9-fused enediyne scaffold (t½ = 68 min);[4] Zein et al. independently remark that deoxyribose 4'-hydrogen abstraction is most likely operative in kedarcidin chromophore bioactivity.
[6] In 2007, Myers and co-workers at Harvard University reported the synthesis of C10-epi-kedarcidin chromophore, corresponding to the 1997 revised structure advanced by Hirama et al. Critical to the success of this endeavor was retrosynthetic analysis that focused on the convergent coupling of components with roughly equal chemical complexity.
In the first incarnation, hydride delivery to a cyclic tetrayne was guided by aluminum coordination to a proximal alkoxide, thus generating the desired enediyne core in one step via two successive 5-exo-dig–type cyclizations.
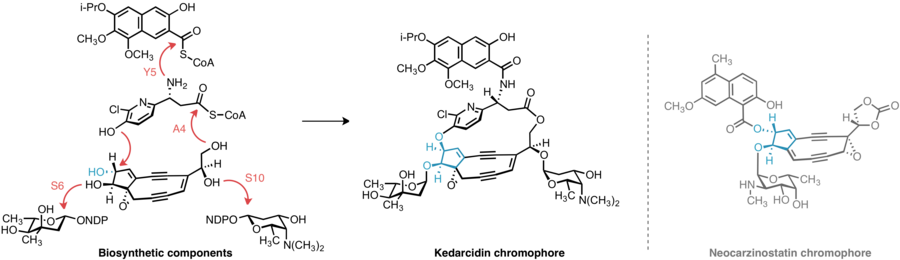
Kedarcidin chromophore, beyond the carbocyclic core it shares with other enediynes, presents additional biosynthetic puzzles: The relative stereochemistry of the groups appended to the carbocyclic core of kedarcidin chomophore differs from that of closely related enediynes; the (R)-2-aza-3-chloro-β-tyrosine substructure has not been identified in any other known natural product; and despite its seeming simplicity, little literature precedence exists for the biosynthesis of the isopropoxy substituent of the naphthonate group.
A convergent biosynthetic strategy is then employed by the producing organisms, whereby the varying peripheral appendages of the enediynes are attached to the core structure to furnish the final product.
The 2-aza-β-tyrosine subunit of kedarcidin chromophore is altogether unknown in any other natural product; this lack of precedence frustrates any attempt at a priori identification of the genes responsible for synthesizing this structure.
[17] Insight into the biosynthesis of the isopropoxy-2-naphthonate appendage was similarly gained by comparative analysis of the ked cluster to those of neocarzinostatin and maduropeptin, enediynes with naphthonate or benzoate substructures, respectively.
[17] Owing to its non-specific cytotoxicity, instability under ambient conditions, and relative expense of isolation and manufacture, kedarcidin chromophore has not been investigated rigorously as a therapeutic candidate.
However, the recent scientific advances discussed above have served to diminish this last hurdle, as fully synthetic and biosynthetic routes toward scalable kedarcidin production are now within reach.
Thus, the biological potential and complex molecular architecture of kedarcidin may likely inspire further scientific inquiry into this substance, and possibly deliver new ordnance in the war against cancer.

![Equilibrium of kedarcidin chromophore core and Bergman-cycloaromatized biradical.[4]](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ef/Kedarcidin_core_Bergman_equilibrium.png/700px-Kedarcidin_core_Bergman_equilibrium.png)


![Ring strain associated with the C1-C12 double bond in kedarcidin chromophore core.[4]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/Ring_strain_MM2.png/250px-Ring_strain_MM2.png)