Energy
Energy (from Ancient Greek ἐνέργεια (enérgeia) 'activity') is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light.
In contrast to the modern definition, energeia was a qualitative philosophical concept, broad enough to include ideas such as happiness and pleasure.
To account for slowing due to friction, Leibniz theorized that thermal energy consisted of the motions of the constituent parts of matter, although it would be more than a century until this was generally accepted.
Writing in the early 18th century, Émilie du Châtelet proposed the concept of conservation of energy in the marginalia of her French language translation of Newton's Principia Mathematica, which represented the first formulation of a conserved measurable quantity that was distinct from momentum, and which would later be called "energy".
These developments led to the theory of conservation of energy, formalized largely by William Thomson (Lord Kelvin) as the field of thermodynamics.
Thermodynamics aided the rapid development of explanations of chemical processes by Rudolf Clausius, Josiah Willard Gibbs, and Walther Nernst.
It also led to a mathematical formulation of the concept of entropy by Clausius and to the introduction of laws of radiant energy by Jožef Stefan.
It is a derived unit that is equal to the energy expended, or work done, in applying a force of one newton through a distance of one metre.
Other energy units such as the electronvolt, food calorie or thermodynamic kcal (based on the temperature change of water in a heating process), and BTU are used in specific areas of science and commerce.
The classical equations of motion can be written in terms of the Hamiltonian, even for highly complex or abstract systems.
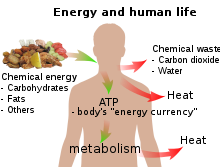
The daily 1500–2000 Calories (6–8 MJ) recommended for a human adult are taken as food molecules, mostly carbohydrates and fats, of which glucose (C6H12O6) and stearin (C57H110O6) are convenient examples.
The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ecology.
Sunlight may be stored as gravitational potential energy after it strikes the Earth, as (for example when) water evaporates from oceans and is deposited upon mountains (where, after being released at a hydroelectric dam, it can be used to drive turbines or generators to produce electricity).
An example of a solar-mediated weather event is a hurricane, which occurs when large unstable areas of warm ocean, heated over months, suddenly give up some of their thermal energy to power a few days of violent air movement.
In cosmology and astronomy the phenomena of stars, nova, supernova, quasars and gamma-ray bursts are the universe's highest-output energy transformations of matter.
Energy in such transformations is either from gravitational collapse of matter (usually molecular hydrogen) into various classes of astronomical objects (stars, black holes, etc.
The nuclear fusion of hydrogen in the Sun also releases another store of potential energy which was created at the time of the Big Bang.
At that time, according to theory, space expanded and the universe cooled too rapidly for hydrogen to completely fuse into heavier elements.
The Schrödinger equation describes the space- and time-dependence of a slowly changing (non-relativistic) wave function of quantum systems.
The solution of this equation for a bound system is discrete (a set of permitted states, each characterized by an energy level) which results in the concept of quanta.
If one (unrealistically) assumes that there is no friction or other losses, the conversion of energy between these processes would be perfect, and the pendulum would continue swinging forever.
The formula E = mc2, derived by Albert Einstein (1905) quantifies the relationship between relativistic mass and energy within the concept of special relativity.
In different theoretical frameworks, similar formulas were derived by J.J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass–energy equivalence#History for further information).
For example, conversion of energy from one type of potential field to another is reversible, as in the pendulum system described above.
As the universe evolves with time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or as other kinds of increases in disorder).
It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number and when we finish watching nature go through her tricks and calculate the number again, it is the same.Most kinds of energy (with gravitational energy being a notable exception)[16] are subject to strict local conservation laws as well.
Virtual photons are also responsible for the electrostatic interaction between electric charges (which results in Coulomb's law), for spontaneous radiative decay of excited atomic and nuclear states, for the Casimir force, for the Van der Waals force and some other observable phenomena.
, can sometimes be ignored, especially for fast processes involving gases, which are poor conductors of heat, or when the thermal efficiency of the transfer is high.
This equation is highly specific, ignoring all chemical, electrical, nuclear, and gravitational forces, effects such as advection of any form of energy other than heat and PV-work.
This principle is vitally important to understanding the behavior of a quantity closely related to energy, called entropy.