Epoxide
This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers.
In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
Some names emphasize the presence of the epoxide functional group, as in the compound 1,2-epoxyheptane, which can also be called 1,2-heptene oxide.
[2] Aside from ethylene oxide, most epoxides are generated when peroxidized reagents donate a single oxygen atom to an alkene.
Safety considerations weigh on these reactions because organic peroxides are prone to spontaneous decomposition or even combustion.
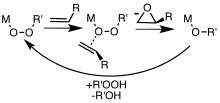
[6] Metal complexes are useful catalysts for epoxidations involving hydrogen peroxide and alkyl hydroperoxides.
[9] Electron-deficient olefins, such as enones and acryl derivatives can be epoxidized using nucleophilic oxygen compounds such as peroxides.
This carbanion then attacks the same oxygen atom, displacing a leaving group from it, to close the epoxide ring.
Peroxycarboxylic acids, which are more electrophilic than other peroxides, convert alkenes to epoxides without the intervention of metal catalysts.
In specialized applications, dioxirane reagents (e.g. dimethyldioxirane) perform similarly, but are more explosive.
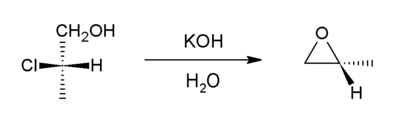
Depending on the mechanism of the reaction and the geometry of the alkene starting material, cis and/or trans epoxide diastereomers may be formed.
The butterfly mechanism allows ideal positioning of the O−O sigma star orbital for C−C π electrons to attack.
[14] Because two bonds are broken and formed to the epoxide oxygen, this is formally an example of a coarctate transition state.
Many metal complexes give active catalysts, and the most important involve titanium, vanadium, or molybdenum.
For prochiral arenes (naphthalene, toluene, benzoates, benzopyrene), the epoxides are often obtained in high enantioselectivity.
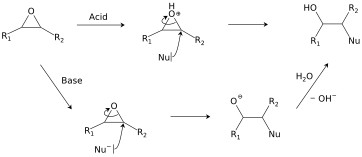
Epoxides react with a broad range of nucleophiles, for example, alcohols, water, amines, thiols, and even halides.
[24] Ring-opening regioselectivity in asymmetric epoxides generally follows the SN2 pattern of attack at the least-substituted carbon,[25] but can be affected by carbocation stability under acidic conditions.
The reaction of an alcohol or a phenol with ethylene oxide, ethoxylation, is widely used to produce surfactants:[28] With anhydrides, epoxides give polyesters.
The reaction of epoxides with amines is the basis for the formation of epoxy glues and structural materials.