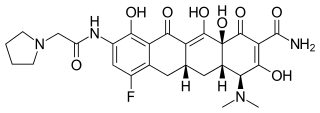
Eravacycline
Eravacycline has shown broad spectrum of activity against a variety of Gram-positive and Gram-negative bacteria, including multi-drug resistant strains, such as methicillin-resistant Staphylococcus aureus (MRSA) and carbapenem-resistant Enterobacteriaceae.
The study enrolled 500 adult patients with the primary endpoint being clinical response at the test-of-cure visit which is 25–31 days after initial dosing.
[12][13] In July 2017, Tetraphase pharmaceuticals released top line data via press showing clinical cure rates in the micro-ITT population to be 90.8% and 91.2% for eravacycline (n=195) and meropenem (n=205), respectively (95% CI: -6.3%,5.3%).
Primary analysis was conducted using a 12.5% non-inferiority margin of the modified intent-to-treat (MITT) and clinically evaluable (CE) patient populations.
Full data from IGNITE4 will become available as the company prepares to submit its New Drug Application (NDA) in the first quarter of 2018 for Eravacycline treatment of Complicated Intra-abdominal Infections.
[14] Due to the performance of the IV formulation, an additional phase 3 trial is planned to support a supplemental NDA for the cUTI indication.
IGNITE3 is currently enrolling approximately 1,000 patients who will be randomized 1:1 to receive intravenous eravacycline or ertapenem for a minimum of 5 days, and will then be eligible for transition to oral levofloxacin.