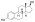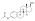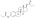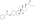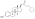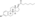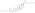Estradiol cypionate
[31][25] Estradiol cypionate is usually used at a dosage of 1 to 5 mg by intramuscular injection every 3 to 4 weeks in the treatment of menopausal symptoms such as hot flashes and vaginal atrophy, at a dosage of 1.5 to 2 mg by intramuscular injection once a month in the treatment of female hypoestrogenism due to hypogonadism, and at a dosage of 2 to 10 mg by intramuscular injection once every 1 or 2 weeks for hormone therapy in transgender women.
[14][17][29][28][32] The doses used to induce puberty in girls are 0.2 to 2.5 mg per month, gradually increased over a period of 4 years.
[26] In addition to single-drug formulations, estradiol cypionate has been marketed in combination with medroxyprogesterone acetate as a microcrystalline aqueous suspension (brand name Lunelle) and in combination with testosterone cypionate as an oil solution (brand name Depo-Testadiol).
Examples of such side effects include breast tenderness and enlargement, nausea, vomiting, bloating, edema, headache, migraine, and melasma.
[60] As such, it appears that similarly to depot medroxyprogesterone acetate, combined injectable contraceptives with 5 mg estradiol cypionate and 25 mg medroxyprogesterone acetate have less or no procoagulant effect relative to combined birth control pills.
[8] The slow release of estradiol cypionate from the tissue depot is caused by the high lipophilicity of the estradiol ester, which in turn is due to its long fatty acid cypionic acid ester moiety.
[26][33][34][35] Aqueous suspensions of steroid esters generally have longer durations by intramuscular injection than oil solutions.
[11] This is because estradiol cypionate has a more extensive fatty acid chain and in relation to this is comparatively more lipophilic.
[8] Estradiol cypionate/medroxyprogesterone acetate (brand names Lunelle, Cyclofem) is a combined injectable contraceptive containing 5 mg estradiol cypionate and 25 mg medroxyprogesterone acetate in microcrystalline aqueous suspension for once-monthly intramuscular administration.
[33] It was first introduced for medical use by Upjohn in 1952 under the brand name Depo-Estradiol in the United States.
[21] When estradiol cypionate was to be combined with medroxyprogesterone acetate as a once-a-month injectable contraceptive, there was a problem in that estradiol cypionate was prepared as an oil solution while medroxyprogesterone acetate was used as a microcrystalline aqueous suspension.
[72][73] Estradiol cypionate has been marketed under the brand names Cicloestradiolo, D-Est, depGynogen, Depo-Estradiol, Depoestra, Depofemin, Depogen, Dura-Estrin, E-Cypionate, E-Ionate, Estradep, Estro-Cyp, Estrofem, Estroject, Estromed-PA, Estronol, Femovirin, Neoginon Depositum, Oestradiol-Retard, Pertradiol, Spendepiol, and T-E Cypionate, among others.
[26][23][25] It was previously marketed in Spain and Italy, but was discontinued in these countries and is no longer available in Europe.
[22][24] Besides the United States, estradiol cypionate has been marketed in France, Germany, Italy, Spain, and Japan, among other countries.
[33][20][22] Estradiol cypionate for human use is not available in Canada, although it is marketed in several veterinary formulations in this country.