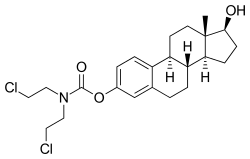
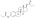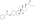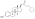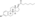Estramustine phosphate
[7][8][3][1][5][12] Side effects of EMP include nausea, vomiting, gynecomastia, feminization, demasculinization, sexual dysfunction, blood clots, and cardiovascular complications.
[18] Due to its relatively severe side effects and toxicity, EMP has rarely been used in the treatment of prostate cancer.
[3] However, encouraging clinical research findings resulted in renewed interest of EMP for the treatment of prostate cancer.
[1] EMP and other estrogens such as polyestradiol phosphate and ethinylestradiol are far less costly than newer therapies such as GnRH modulators, abiraterone acetate, and enzalutamide.
[4][21][22] In addition, estrogens may offer significant benefits over other means of androgen deprivation therapy, for instance in terms of bone loss and fractures, hot flashes, cognition, and metabolic status.
[4][22] EMP has been used to prevent the testosterone flare at the start of GnRH agonist therapy in men with prostate cancer.
[19][24][25][7] EMP is contraindicated when used in children, patients hypersensitive to estrogens or nitrogen mustards, those with peptic ulcer (an ulcer in the digestive tract), those with severely compromised liver function, those with weak heart muscle (also known as myocardial insufficiency) and those with thromboembolic disorders or complications related to fluid retention.
[9] Nonetheless, severe cases of gastrointestinal side effects with EMP may require dose reduction or discontinuation of therapy.
[3] As a rule, feminization, a gynoid fat distribution, demasculinization, and impotence are said to occur in virtually or nearly 100% of men treated with high-dose estrogen therapy.
[13] Severe adverse effects of EMP are thromboembolic and cardiovascular complications including pulmonary embolism, deep vein thrombosis, stroke, thrombophlebitis, coronary artery disease (ischemic heart disease; e.g., myocardial infarction), thrombophlebitis, and congestive heart failure with fluid retention.
[29] Anticoagulant therapy with medications such as aspirin, warfarin, unfractionated and low-molecular-weight heparin, and vitamin K antagonists can be useful for decreasing the risk of thromboembolism with EMP and other estrogens like diethylstilbestrol and ethinylestradiol.
[32][33] In a small low-dose study using 280 mg/day oral EMP for 150 days, tolerability was significantly improved, with gastrointestinal irritation occurring in only 15% of men, and there was no incidence of severe cardiovascular toxicity or deep vein thrombosis.
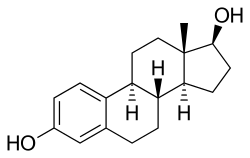
[35][15] Because EMP is a prodrug of estradiol, it may be considered to be a natural and bioidentical form of estrogen,[14] although it does have additional cytostatic activity via estramustine and estromustine.
[38] Metabolites of EMP, including estramustine, estromustine, estradiol, and estrone, have been found to act as weak antagonists of the androgen receptor (EC50Tooltip half-maximal effective concentration = 0.5–3.1 μM), although the clinical significance of this is unknown.
[5][31] However, it has shown essentially the same rates and degrees of estrogenic effects, such as breast tenderness, gynecomastia, cardiovascular toxicity, changes in liver protein synthesis, and testosterone suppression, as high-dose diethylstilbestrol and ethinylestradiol in clinical studies.
[1] EMP is considered to mainly be a mitotic inhibitor, inhibiting mechanisms involved in the mitosis phase of the cell cycle.
[1][3][43] As such, the unique properties of the estramustine and estromustine structures, containing a carbamate-ester bond, appear to be responsible for the cytostatic effects of EMP.
[1] In addition to its antimitotic actions, EMP has also been found to produce other cytostatic effects, including induction of apoptosis, interference with DNA synthesis, nuclear matrix interaction, cell membrane alterations, induction of reactive oxygen species (free oxygen radicals), and possibly additional mechanisms.
[1][4] EMP has been found to have a radiosensitizing effect in prostate cancer and glioma cells, improving sensitivity to radiation therapy as well.
[1] The cytostatic metabolites of EMP are accumulated in tissues in a selective manner, for instance in prostate cancer cells.
[4] However, subsequent research found that there is very limited and slow cleavage of the normustine ester and that EMP is devoid of alkylating activity.
[1][3] Extremely high, pregnancy-like levels of estradiol may be responsible for the leukocytosis (increased white blood cell count) that is observed in individuals treated with EMP.
[3] Upon oral ingestion, EMP is rapidly and completely dephosphorylated by phosphatases into estramustine during the first pass in the gastrointestinal tract.
[1][12] A limited quantity of approximately 10 to 15% of estramustine and estromustine is further slowly metabolized via hydrolysis of the normustine ester into estradiol and estrone, respectively.
[43][49] Release of nitrogen mustard gas from normustine via cleavage of the carboxylic acid group has not been demonstrated and does not seem to occur.
[1] Consumption of calcium, aluminium, or magnesium with oral EMP can markedly impair its bioavailability due to diminished absorption from the intestines, and this may interfere with its therapeutic effectiveness at low doses.
[3][17] Following a single oral dose of 420 mg EMP in men with prostate cancer, maximal levels of estromustine were 310 to 475 ng/mL (475,000 pg/mL) and occurred after 2 to 3 hours.
[1][31] Nonetheless, estradiol levels during EMP therapy appear to be similar to those that occur in mid-to-late pregnancy, which range from 5,000 to 40,000 pg/mL.
[35][15] Antineoplastic agents related to EMP, although none of them were marketed, include alestramustine, atrimustine, cytestrol acetate, estradiol mustard, ICI-85966, and phenestrol.
[19] The phosphate ester of EMP was incorporated into the molecule in order to increase its water solubility and allow for intravenous administration.