Estrogen receptor
Upon activation by estrogen, intracellular ERs undergo translocation to the nucleus where they bind to specific DNA sequences.
These receptors mediate the effects of their respective hormones, contributing to the development and maintenance of reproductive functions and secondary sexual characteristics.
In humans, the two forms of the estrogen receptor are encoded by different genes, ESR1 and ESR2 on the sixth and fourteenth chromosome (6q25.1 and 14q23.2), respectively.
There are two different forms of the estrogen receptor, usually referred to as α and β, each encoded by a separate gene (ESR1 and ESR2, respectively).
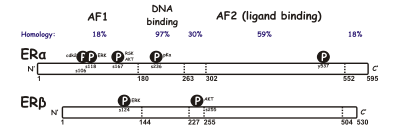
[citation needed] The N-terminal A/B domain is able to transactivate gene transcription in the absence of bound ligand (e.g., the estrogen hormone).
[12] Since estrogen is a steroidal hormone, it can readily diffuse through the phospholipid membranes of cells due to its lipophilic nature.
Finally, the receptor dimer binds to specific DNA sequences known as hormone response elements, initiating the process of gene regulation.
[15] Tumor suppressor kinase LKB1 coactivates ERα in the cell nucleus through direct binding, recruiting it to the promoter of ERα-responsive genes.
[17] Direct acetylation of estrogen receptor alpha at lysine residues in the hinge region by p300 regulates transactivation and hormone sensitivity.
[24] Estrogen receptors are over-expressed in around 70% of breast cancer cases, referred to as "ER-positive", and can be demonstrated in such tissues using immunohistochemistry.
Two hypotheses have been proposed to explain why this causes tumorigenesis, and the available evidence suggests that both mechanisms contribute: The result of both processes is disruption of cell cycle, apoptosis and DNA repair, which increases the chance of tumour formation.
[27] Another SERM, raloxifene, has been used as a preventive chemotherapy for women judged to have a high risk of developing breast cancer.
[28] Another chemotherapeutic anti-estrogen, ICI 182,780 (Faslodex), which acts as a complete antagonist, also promotes degradation of the estrogen receptor.
[29] Massively parallel genome sequencing has revealed the common presence of point mutations on ESR1 that are drivers for resistance, and promote the agonist conformation of ERα without the bound ligand.
[8] A dramatic demonstration of the importance of estrogens in the regulation of fat deposition comes from transgenic mice that were genetically engineered to lack a functional aromatase gene.
[37][38] The ER's helix 12 domain plays a crucial role in determining interactions with coactivators and corepressors and, therefore, the respective agonist or antagonist effect of the ligand.
[43] The concept of selective estrogen receptor modulators is based on the ability to promote ER interactions with different proteins such as transcriptional coactivator or corepressors.
[47] The gene for a second estrogen receptor (ERβ) was identified in 1996 by Kuiper et al. in rat prostate and ovary using degenerate ERalpha primers.