Ethylene glycol
External SDS 2 Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol[7]) with the formula (CH2OH)2.
The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water.
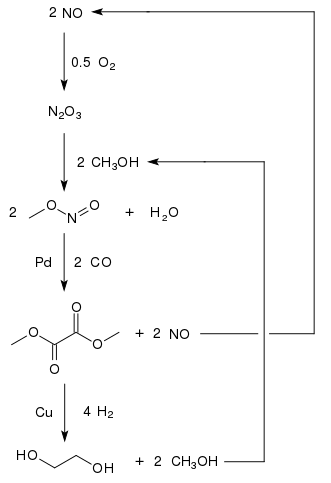
Ethylene glycol is produced from carbon monoxide in countries with large coal reserves and less stringent environmental regulations.
The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to the production of C1-based ethylene glycol.
The major use of ethylene glycol is as an antifreeze agent in the coolant in for example, automobiles and air-conditioning systems that either place the chiller or air handlers outside or must cool below the freezing temperature of water.
The mixture of ethylene glycol with water provides additional benefits to coolant and antifreeze solutions, such as preventing corrosion and acid degradation, as well as inhibiting the growth of most microbes and fungi.
[24] Because of the depressed freezing temperatures, ethylene glycol is used as a de-icing fluid for windshields and aircraft, as an antifreeze in automobile engines, and as a component of vitrification (anticrystallization) mixtures for low-temperature preservation of biological tissues and organs.
Ethylene glycol can be recovered from the natural gas and reused as an inhibitor after purification treatment that removes water and inorganic salts.
In this application, ethylene glycol flows down from the top of a tower and meets a rising mixture of water vapor and hydrocarbon gases.
Minor uses of ethylene glycol include the manufacture of capacitors, as a chemical intermediate in the manufacture of 1,4-dioxane, as an additive to prevent corrosion in liquid cooling systems for personal computers, and inside the lens devices of cathode-ray tube type of rear projection televisions.
Ethylene glycol is commonly used as a preservative for biological specimens, especially in secondary schools during dissection as a safer alternative to formaldehyde.
In one example, isophorone was protected using ethylene glycol:[27] The glycol-derived dioxalane of ethyl acetoacetate is a commercial fragrance fructone.
[28] Silicon dioxide dissolves slowly in hot ethylene glycol in the presence of alkali metal base to produce silicates.
[29] Ethylene glycol has relatively high mammalian toxicity when ingested, roughly on par with methanol, with an oral LDLo = 786 mg/kg for humans.
They are generally considered safer to use, as propylene glycol is not as palatable[note 1] and is converted in the body to lactic acid, a normal product of metabolism and exercise.
[35] Australia, the UK, and seventeen US states (as of 2012) require the addition of a bitter flavoring (denatonium benzoate) to antifreeze.
[36] In 2022, several hundred children died of acute kidney failure in Indonesia and The Gambia because the paracetamol syrup made by New Delhi-based Maiden Pharmaceuticals contained ethylene glycol and diethylene glycol, ingredients that have been linked to child deaths from acute kidney injury in The Gambia.
[37] In December 2022, Uzbekistan's health ministry has said children died as a result of ethylene glycol in cough syrup made by Marion Biotech, which is based at Noida, near New Delhi.
It enters the environment through the dispersal of ethylene glycol-containing products, especially at airports, where it is used in de-icing agents for runways and airplanes.
"Based on a rather extensive database, it induces skeletal variations and malformations in rats and mice by all routes of exposure.