Ethenone
It must therefore always be prepared for each use and processed immediately, otherwise a dimerization to diketene occurs or it reacts to polymers that are difficult to handle.
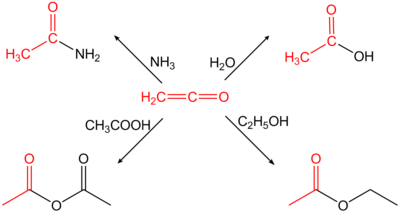
[6] Because of its cumulative double bonds, ethenone is highly reactive and reacts in an addition reaction H-acidic compounds to the corresponding acetic acid derivatives.
[12][13][14] Ethenone was discovered at the same time by Hermann Staudinger (by reaction of bromoacetyl bromide with metallic zinc)[15][16] The dehydration of acetic acid was reported in 1910.
[27] Exposure to concentrated levels causes humans to experience irritation of body parts such as the eye, nose, throat and lungs.
Extended toxicity testing on mice, rats, guinea pigs and rabbits showed that ten-minute exposures to concentrations of freshly generated ethenone as low as 0.2 mg/liter (116 ppm) may produce a high percentage of deaths in small animals.
[28][20] The formation of ketene in the pyrolysis of vitamin E acetate, an additive of some e-liquid products, is one possible mechanism of the reported pulmonary damage[29] caused by electronic cigarette use.
[33] An IDLH limit is set at 5 ppm, as this is the lowest concentration productive of a clinically relevant physiologic response in humans.