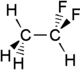
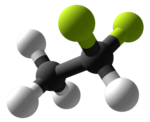
1,1-Difluoroethane
[6] In addition, 1,1-difluoroethane is also commonly used in gas dusters and numerous other retail aerosol products, particularly those subject to stringent volatile organic compound (VOC) requirements.
The molecular weight of difluoroethane is 66, making it a useful and convenient tool for detecting vacuum leaks in Gas chromatography–mass spectrometry (GC-MS) systems.
Difluoroethane is an extremely flammable gas, which decomposes rapidly on heating or burning, producing toxic and irritating fumes, including hydrogen fluoride and carbon monoxide.
[14][15] Fatalities linked to difluoroethane abuse include actress Skye McCole Bartusiak, singer Aaron Carter and wrestler Mike Bell.
[16] Bitterants, added voluntarily to some brands to deter purposeful inhalation, are often not legally required; they do not negate or counteract difluoroethane's intoxicating effects.