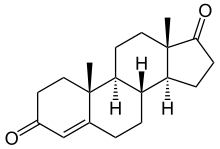
Exemestane
[4] Exemestane is also indicated for the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.
[7] The most common side effects (more than 10% of patients) are hot flashes and sweating, which are typical of estrogen deficiency as caused by exemestane, and also insomnia, headache, and joint pain.
No life-threatening overdosing is known in humans, but only in animal studies with 2000- to 4000-fold doses (adjusted to body surface area).
While the CYP3A4 inhibitor ketoconazole had no significant effect on exemestane levels in a clinical trial, the strong CYP3A4 inductor rifampicin significantly cut exemenstane levels about in half (AUC −54%, Cmax −41% for a single dose), potentially compromising its effectiveness.
Exemestane is an oral steroidal aromatase inhibitor that is used in ER-positive breast cancer in addition to surgery and/or radiation in post-menopausal women.
[12] Exemestane is an irreversible, steroidal aromatase inactivator of type I, structurally related to the natural substrate 4-androstenedione.
[11] A study conducted on young adult males found that the estrogen suppression rate for exemestane varied from 35% for estradiol (E2) to 70% for estrone (E1).
[13] Exemestane is quickly absorbed from the gut, but undergoes a strong first-pass effect in the liver.
The drug also counteracts gynecomastia as well as fat and water retention following excessive aromatase production due to testosterone doping.
Rarely, it is used recreationally by teenagers to delay epiphyseal plate closure and increase adult height, particularly among members of the Looksmaxxing and incel communities, where its use is documented on their forums.
[20] Oral exemestane 25 mg/day for 2–3 years of adjuvant therapy was generally more effective than 5 years of continuous adjuvant tamoxifen in the treatment of postmenopausal women with early-stage estrogen receptor-positive/unknown receptor status breast in a large well-designed[citation needed] trial.
In 4,560 women, after 35 months, the administration of exemestane at a dose of 25 mg/day resulted in a 65% reduction in the risk of breast cancer compared with placebo; annual incidence rates were 0.19% and 0.55%, respectively (hazard ratio: 0.35; 95% CI [0.18-0.70]; p = 0.002).