Exocytosis
In exocytosis, membrane-bound secretory vesicles are carried to the cell membrane, where they dock and fuse at porosomes and their contents (i.e., water-soluble molecules) are secreted into the extracellular environment.
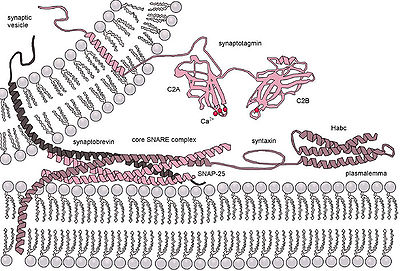
The calcium sensors that trigger exocytosis might interact either with the SNARE complex or with the phospholipids of the fusing membranes.
There is no clear consensus about the machinery and molecular processes that drive the formation, budding, translocation and fusion of the post-Golgi vesicles to the plasma membrane.
This finding of membrane vesicle trafficking occurring at the host–pathogen interface also dispels the myth that exocytosis is purely a eukaryotic cell phenomenon.
Tethering involves links over distances of more than about half the diameter of a vesicle from a given membrane surface (>25 nm).
Secretory vesicles transiently dock and fuse at the porosome at the cell plasma membrane, via a tight t-/v-SNARE ring complex.
In neuronal exocytosis, the term priming has been used to include all of the molecular rearrangements and ATP-dependent protein and lipid modifications that take place after initial docking of a synaptic vesicle but before exocytosis, such that the influx of calcium ions is all that is needed to trigger nearly instantaneous neurotransmitter release.
In other cell types, whose secretion is constitutive (i.e. continuous, calcium ion independent, non-triggered) there is no priming.
The merging of the donor and the acceptor membranes accomplishes three tasks: Retrieval of synaptic vesicles occurs by endocytosis.
Non-constitutive exocytosis and subsequent endocytosis are highly energy expending processes, and thus, are dependent on mitochondria.
This could only be possible if the vesicle were to temporarily establish continuity with the cell plasma membrane at porosomes, expel a portion of its contents, then detach, reseal, and withdraw into the cytosol (endocytose).
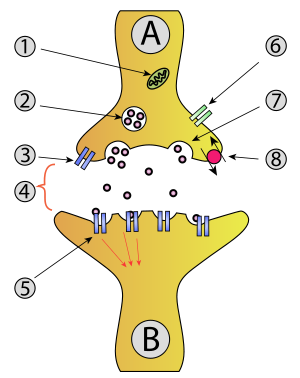
- Mitochondrion
- Synaptic vesicle with neurotransmitters
- Autoreceptor
- Synapse with neurotransmitter released ( serotonin )
- Postsynaptic receptors activated by neurotransmitter (induction of a postsynaptic potential)
- Calcium channel
- Exocytosis of a vesicle
- Recaptured neurotransmitter