Positron emission tomography
Positron emission tomography (PET)[1] is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption.
PET is a valuable research tool to learn and enhance our knowledge of the normal human brain, heart function, and support drug development.
PET scanning does this by using radiolabelled molecular probes that have different rates of uptake depending on the type and function of tissue involved.
Regional tracer uptake in various anatomic structures can be visualized and relatively quantified in terms of injected positron emitter within a PET scan.
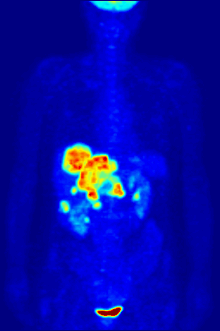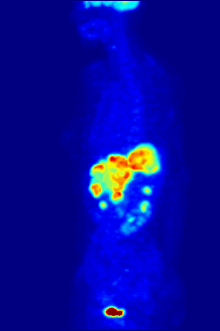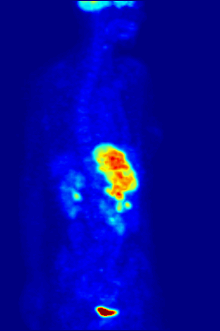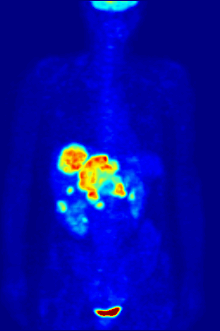
FDG is a glucose analog that is taken up by glucose-using cells and phosphorylated by hexokinase (whose mitochondrial form is significantly elevated in rapidly growing malignant tumors).
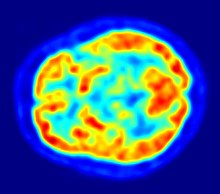
The concentrations of imaged FDG tracer indicate tissue metabolic activity as it corresponds to the regional glucose uptake.
These probes permit the visualization of amyloid plaques in the brains of Alzheimer's patients and could assist clinicians in making a positive clinical diagnosis of AD pre-mortem and aid in the development of novel anti-amyloid therapies.
[11C]polymethylpentene (PMP) is a novel radiopharmaceutical used in PET imaging to determine the activity of the acetylcholinergic neurotransmitter system by acting as a substrate for acetylcholinesterase.
[26] Avid Radiopharmaceuticals has developed and commercialized a compound called florbetapir that uses the longer-lasting radionuclide fluorine-18 to detect amyloid plaques using PET scans.
Studies have been performed examining the state of these receptors in patients compared to healthy controls in schizophrenia, substance abuse, mood disorders and other psychiatric conditions.
[citation needed] PET can also be used in image guided surgery for the treatment of intracranial tumors, arteriovenous malformations and other surgically treatable conditions.
The information regarding drug uptake, retention and elimination over time can be obtained quickly and cost-effectively compare to the older technique of killing and dissecting the animals.
The scanners are based on microminiature scintillators and amplified avalanche photodiodes (APDs) through a system that uses single-chip silicon photomultipliers.
Because of cost as well as the marginal utility of detecting cancer metastases in companion animals (the primary use of this modality), veterinary PET scanning is expected to be rarely available in the immediate future.
[3] FDG, which is now the standard radiotracer used for PET neuroimaging and cancer patient management,[40] has an effective radiation dose of 14 mSv.
Due to the short half-lives of most positron-emitting radioisotopes, the radiotracers have traditionally been produced using a cyclotron in close proximity to the PET imaging facility.
The emitted positron travels in tissue for a short distance (typically less than 1 mm, but dependent on the isotope[54]), during which time it loses kinetic energy, until it decelerates to a point where it can interact with an electron.
[56] The raw data collected by a PET scanner are a list of 'coincidence events' representing near-simultaneous detection (typically, within a window of 6 to 12 nanoseconds of each other) of annihilation photons by a pair of detectors.
These algorithms compute an estimate of the likely distribution of annihilation events that led to the measured data, based on statistical principles.
The advantage is a better noise profile and resistance to the streak artifacts common with FBP, but the disadvantage is greater computer resource requirements.
Time-of-flight PET makes use of very fast gamma-ray detectors and data processing system which can more precisely decide the difference in time between the detection of the two photons.
[73] For brain imaging, registration of CT, MRI and PET scans may be accomplished without the need for an integrated PET-CT or PET-MRI scanner by using a device known as the N-localizer.
Few hospitals and universities are capable of maintaining such systems, and most clinical PET is supported by third-party suppliers of radiotracers that can supply many sites simultaneously.
[79] Because the half-life of fluorine-18 is about two hours, the prepared dose of a radiopharmaceutical bearing this radionuclide will undergo multiple half-lives of decay during the working day.
This necessitates frequent recalibration of the remaining dose (determination of activity per unit volume) and careful planning with respect to patient scheduling.
[80] In the 1960s and 70s tomographic imaging instruments and techniques were further developed by Michel Ter-Pogossian, Michael E. Phelps, Edward J. Hoffman and others at Washington University School of Medicine.
[81][82] Work by Gordon Brownell, Charles Burnham and their associates at the Massachusetts General Hospital beginning in the 1950s contributed significantly to the development of PET technology and included the first demonstration of annihilation radiation for medical imaging.
In particular, the development of labeled 2-fluorodeoxy-D-glucose (FDG-firstly synthethized and described by two Czech scientists from Charles University in Prague in 1968)[85] by the Brookhaven group under the direction of Al Wolf and Joanna Fowler was a major factor in expanding the scope of PET imaging.
Although many investigators took this approach, James Robertson[88] and Zang-Hee Cho[89] were the first to propose a ring system that has become the prototype of the current shape of PET.
[93] In Australia, as of July 2018, the Medicare Benefits Schedule Fee for whole body FDG PET ranges from A$953 to A$999, depending on the indication for the scan.