Fas ligand
[5] Fas ligand or FasL is a type II transmembrane protein belonging to the tumor necrosis factor superfamily (TNFSF).
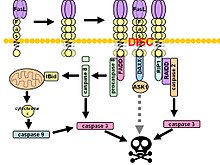
The interaction between FasR on an adjacent cell and membrane anchored FasL leads to the trimerization, forming the death-inducing signaling complex (DISC).
[10] Upon ensuing death domain (DD) aggregation, the receptor complex is internalized via the cellular endosomal machinery.
Active caspase-8 is then released from the DISC into the cytosol, where it cleaves other effector caspases, eventually leading to DNA degradation, membrane blebbing, and other hallmarks of apoptosis.
[11][10] Some reports have suggested that the extrinsic Fas pathway is sufficient to induce complete apoptosis in certain cell types through death-inducing signaling complex (DISC) assembly and subsequent caspase-8 activation.
[10] Additionally, the c-FLIP protein, structurally resembling caspase-8 but lacking enzymatic activity, plays a dual role in Fas-induced apoptosis.
Its functions include: Defective Fas-mediated apoptosis may lead to oncogenesis as well as drug resistance in existing tumors.