Fas receptor
The gene lies on the plus (Watson strand) and is 25,255 bases in length organized into nine protein encoding exons.
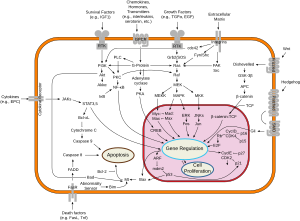
[citation needed] Fas forms the death-inducing signaling complex (DISC) upon ligand binding.
[citation needed] Upon ensuing death domain (DD) aggregation, the receptor complex is internalized via the cellular endosomal machinery.
Active caspase-8 is then released from the DISC into the cytosol, where it cleaves other effector caspases, eventually leading to DNA degradation, membrane blebbing, and other hallmarks of apoptosis.
In AOM-DSS-induced colon carcinoma and MCA-induced sarcoma mouse models, it has been shown that Fas acts as a tumor suppressor.
CTL-mediated bystander killing was described by the Fleischer Lab in 1986[18] and later attributed to fas-mediated lysis in vitro by the Austin Research Institute, Cellular Cytotoxicity Laboratory.
[21] Some reports have suggested that the extrinsic Fas pathway is sufficient to induce complete apoptosis in certain cell types through DISC assembly and subsequent caspase-8 activation.
[citation needed] In most cell types, caspase-8 catalyzes the cleavage of the pro-apoptotic BH3-only protein Bid into its truncated form, tBid.
BH-3 only members of the Bcl-2 family exclusively engage anti-apoptotic members of the family (Bcl-2, Bcl-xL), allowing Bak and Bax to translocate to the outer mitochondrial membrane, thus permeabilizing it and facilitating release of pro-apoptotic proteins such as cytochrome c and Smac/DIABLO, an antagonist of inhibitors of apoptosis proteins (IAPs).