Ferredoxin
Ferredoxins (from Latin ferrum: iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions.
The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.
It accepts electrons produced from sunlight-excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase EC 1.18.1.2.
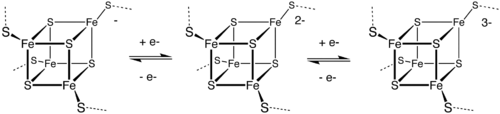
These biological "capacitors" can accept or discharge electrons, with the effect of a change in the oxidation state of the iron atoms between +2 and +3.
However a few bacterial ferredoxins (of the 2[4Fe4S] type) have two iron sulfur clusters and can carry out two electron transfer reactions.
If the cell is growing on substrates that provide excess Fd−red, the Rnf complex can transfer these electrons to NAD+ and store the resultant energy in the membrane potential.
Typically, in oxidative phosphorylation the transfer of electrons from NADH to ubiquinone (Q) is coupled to charging the proton motive force.
[10] Members of the 2Fe–2S ferredoxin superfamily (InterPro: IPR036010) have a general core structure consisting of beta(2)-alpha-beta(2), which includes putidaredoxin, terpredoxin, and adrenodoxin.
One group of ferredoxins, originally found in chloroplast membranes, has been termed "chloroplast-type" or "plant-type" (InterPro: IPR010241).
In hydroxylating bacterial dioxygenase systems, they serve as intermediate electron-transfer carriers between reductase flavoproteins and oxygenase.
Although the physiological role of this ferredoxin remains unclear, a strong and specific interaction of Cp2FeFd with the molybdenum-iron protein of nitrogenase has been revealed.
[24] Adrenodoxin (adrenal ferredoxin; InterPro: IPR001055), putidaredoxin, and terpredoxin make up a family of soluble Fe2S2 proteins that act as single electron carriers, mainly found in eukaryotic mitochondria and Pseudomonadota.
[26][27] The exact functions of other members of this family are not known, although Escherichia coli Fdx is shown to be involved in biogenesis of Fe–S clusters.
During the evolution of bacterial-type ferredoxins, intrasequence gene duplication, transposition and fusion events occurred, resulting in the appearance of proteins with multiple iron–sulfur centers.
The fold belongs to the α+β class, with 2-7 α-helices and four β-strands forming a barrel-like structure, and an extruded loop containing three "proximal" Cys ligands of the iron–sulfur cluster.
High potential iron–sulfur proteins (HiPIPs) form a unique family of Fe4S4 ferredoxins that function in anaerobic electron transport chains.