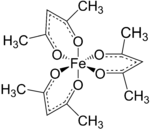
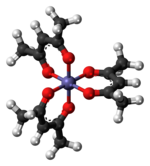
Tris(acetylacetonato)iron(III)
As the metal orbitals are all evenly occupied the complex is not subject to Jahn-Teller distortions and thus adopts a D3 molecular symmetry.
In contrast, the related metal acetylacetonate Mn(acac)3 adopts a more distorted octahedral structure.
[3] The 5 unpaired d-electrons also result in the complex being paramagnetic, with a magnetic moment of 5.90 μB.
[4] Fe(acac)3 has been examined as a precatalyst and reagent in organic chemistry, although the active iron-containing species is usually unidentified in these processes.
[7] Beyond the area of polymerization, Fe(acac)3 has been found to catalyze the reaction of N-sulfonyl oxaziridines with olefins to form 1,3-oxazolidine products.