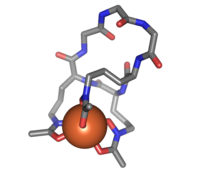
Ferrichrome
The main function of siderophores is to chelate ferric iron (Fe3+) from insoluble minerals from the environment and make it available for microbial and plant cells.
Iron is important in biological functions as it acts as a catalyst in enzymatic processes, as well as for electron transfer, DNA and RNA synthesis, and oxygen metabolism.
[5] Recent studies have shown that ferrichrome has been used as a tumor- suppressive molecule produced by the bacterium Lacticaseibacillus casei.
The study from the Department of Medicine and Asahikawa Medical University, suggests that ferrichrome has a greater tumor-suppressive effect than other drugs currently used to fight colon cancer, including cisplatin and 5-fluoro-uracil.
The induction of apoptosis by ferrichrome is reduced by the inhibition of the c-jun N-terminal kinase signaling pathway.
[7] To overcome this, bacteria, fungi and some plants synthesize siderophores, and secrete it into an extracellular environment where binding of iron can occur.
[7] For example, saccharomyces cerevisiae is a species of yeast that can uptake the iron bound siderophore through transporters of the ARN family.
[10] In terms of the HSAB principle, ferric siderophores have donor atoms that are mainly oxygen and rarely heterocyclic nitrogen.
[12] It is involved in the uptake of iron in complex with ferrichrome by binding and transporting ferrichrome-iron across the cell’s outer membrane.
This energy transfer results in subsequent conformational changes that transport iron-ferrichrome to the periplasmic pocket which signal a ligand loaded status of the receptor.
[12] These subtle shifts disrupt the binding of iron-ferrichrome to the cork which then allows the permeation of the ferrichrome-iron to a putative channel-forming region.
[12] FhuD is a high affinity binding protein in the periplasmic pocket that also aids in unidirectional transport across the cell envelope.