Ferricyanide
The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry.
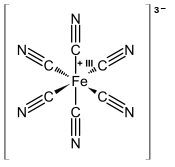
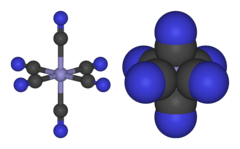
[1] [Fe(CN)6]3− consists of a Fe3+ center bound in octahedral geometry to six cyanide ligands.
The iron is low spin and easily reduced to the related ferrocyanide ion [Fe(CN)6]4−, which is a ferrous (Fe2+) derivative.
They do react with mineral acids, however, to release highly toxic hydrogen cyanide gas.
Treatment of ferricyanide with iron(II) salts affords the brilliant, long-lasting pigment Prussian blue, the traditional color of blueprints.