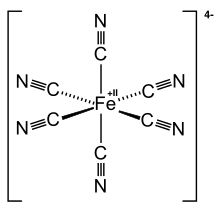
Ferrocyanide
[Fe(CN)6]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment.
[1] It is of commercial interest as a precursor to the pigment Prussian blue and, as its potassium salt, an anticaking agent.
For this reason ferrocyanide has been used as a probe of extracellular electron acceptor in the study of redox reactions in cells.
Ferricyanide is consumed in the process, thus any increase in ferrocyanide can be attributed to secretions of reductants or transplasma membrane electron transport activity.
[4] Aspirational applications range from hydrogen production for cleaner energy with lower CO2 emission to wastewater treatment.